STRUCTURE OF HEMOGLOBIN & MYOGLOBIN
Hemoglobin contains four heme units each embedded in a globular protein sub-unit. There are two types of protein sub-units i.e., α and β.
Myoglobin contains only one heme unit surrounded by a globular protein, containing seven α-helical and six non helical segments, made up of 153 amino acids .
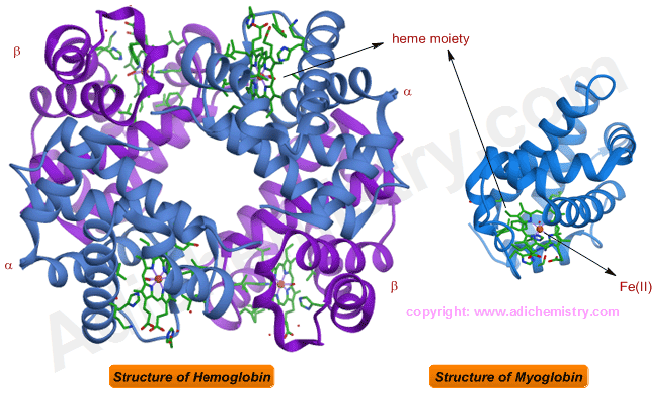
Note: Heme moieties are shown in green color in above diagram. Fe(II) ion is shown in red color.
Heme is a porphyrin ring system made up of four pyrrole rings with an Fe(II) ion coordinated to nitrogens of pyrrole rings.
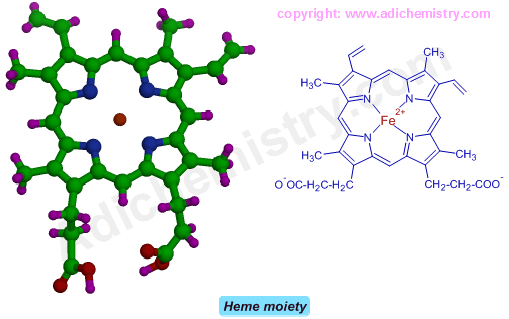
Globin part prevents irreversible oxidation of Fe(II) ion by providing hydrophobic environment. It enhances the selectivity for O2 binding. In hemoglobin, the tetramer arrangement allows for co-operativity by making it more efficient in binding to dioxygen.
Hemoglobin and Myoglobin exist in two forms i.e.,
1) deoxy form: No oxygen is bound to iron.
2) oxy form: dioxygen is bound to iron.
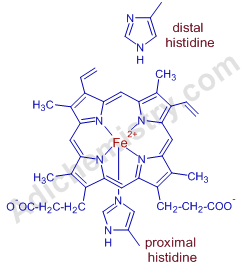
In deoxy-hemoglobin, four of the coordinated sites of iron are occupied by nitrogens of porphyrin ring. The fifth site is occupied by Histidine residue (called proximal histidine) of globin. The sixth position is occupied by weakly bonded water molecule. Hence some authors tend to report Fe(II) ion in deoxy form as pentacoordinated. Deoxy-hemoglobin is said to be in T-state (tense).
On the opposite side of the proximal histidine, there is one more histidine group (called distal histidine) placed near the iron ion. It forces the binding of dioxygen in "end on bent" confirmation.
Note: The bent confirmation discourages the binding of CO to heme iron. Otherwise, CO may have even more affinity with the iron ion. It is observed that CO binds to hemoglobin 200X stronger than dioxygen but binds 20,000X stronger with unprotected heme.
Hemoglobin coordinated to dioxygen is called oxy-hemoglobin. It is also referred to as R-state (relaxed). In oxy-hemoglobin the sixth coordinated position of iron is occupied by dioxygen in "end on bent" geometry.
FUNCTIONING OF HEMOGLOBIN - A BIOINORGANIC PERSPECTIVE
In deoxy-Hemoglobin, the porphyring ring is dome shaped. The Fe(II) is in high spin state and is paramagnetic. Its size is 0.78 Ao and is positioned above the plane of the porphyrin ring.
However, in oxy-Hemoglobin, the size of iron ion is reduced to 0.61 Ao and can fit into the cavity of planar porphyrin ring and hence moves into the cavity of porphyrin ring with concomitant dragging of proximal histidine that triggers the conformational changes in other globin subunits and thus by opening other heme sites. As a result, the binding capacity of other heme irons with dioxygen is enhanced. This is best example for co-operativity through allostery.
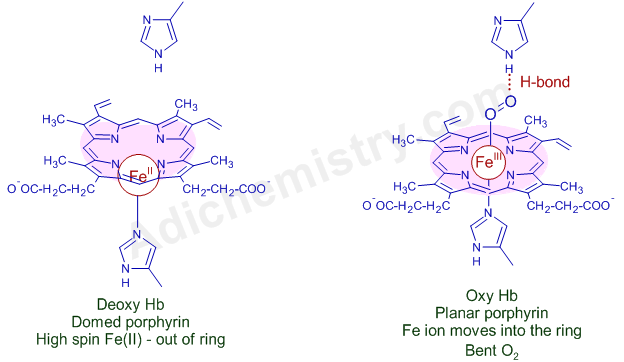
The nature of Fe in oxy-Hemoglobin or in oxy-Myoglobin is controversial.
According to old Pauling model, there is a low spin Fe(II) ion that is bound to singlet O2 in oxy-Hb. Both are diamagnetic.
However, according to Weiss model, there is Fe(III) ion bound to superoxide radical anion (O2-). Though both are paramagnetic, a strong paramagnetic coupling between them ensues diamagnetic behavior . This model is supported by the O-O stretching frequency at 1105 cm-1 in resonance raman spectrum that is consistent with the fact that O2 is in superoxide form. This deems to be more accurate and modern explanation.