a) 25:9
b) 5:3
c) 9:25
d) 3:5
Logic:
The wavelength, λ of electron wave can be expressed by de Broglie's equation:
![]()
It is is related to kinetic energy, KE as follows:
![]()
i.e. KE is inversely proportional to λ2
![]()
Hence the ratio of kinetic energies of electron will be:
KE1: KE2 = λ22 : λ12 = 52 : 32 = 25 : 9
Conclusion:
The correct option is "a".
(EAMCET 2008-E)
a) 2:1
b) 1:4
c) 1:1
d) 4:1
Logic & solution:
From de Broglies equation, the ratio of wavelengths is related to their masses and velocities as follows:
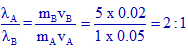
Conclusion:
Correct option is "a".
(EAMCET 2005-E)
1) 2/λ
b) 2λ
c) 4λ
d) -4/λ
Logic & solution:
According to de Broglies concept, the circumference of circle, 2πr must be equal to nλ.
Since n = 4 for fourth orbit,
Circumference, 2πr = 4λ
Conclusion:
Correct option is "c".
(AIEEE 2003)
a) 10-33 m
b) 10-31 m
c) 10-16 m
d) 10-25 m
solution:
λ = h/mv = 6.63x 10-34 J s / 60 x 10-3 kg x 10 m s-1 = 1.1 x 10-33 m
Note: Use only SI units since Planck's constant is in SI units.
Conclusion:
Correct option is "c".
1) What is the ratio of de Broglie's wavelengths of electron and proton moving with same velocities?
2) What is the wavelength of electron moving in 5th orbit of hydrogen atom?
Jump to Photo electric effect - Einstein - IIT JEE - NEET - IT JAM solved problems