(IIT JEE 2001)
1) A4B3
2) AB2
3) A2B
4) A3B4
Logic & Solution:
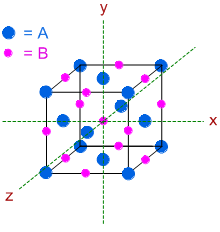
Solid 'AB' has NaCl structure, otherwise known as Rock salt structure. Therefore, same type of atoms (for example 'A' atoms) are arranged in FCC pattern (in which all the 8 corners and all the 6 face centers are occupied) while other type of atoms ('B' atoms) occupy all the 12 edges as well as at the center of the unit cell.
Since, 'A' atoms are occupying the corners of unit cell, they must also be present at the face centers. The B atoms can be found at the 12 edges and at the center of the unit cell as shown below.

Therefore, before removing face centered atoms:
The number of 'A' atoms = (8 x 1/8) + (6 x 1/2) = 1 + 3 = 4 [there are 8 'A' atoms at the corners and 6 'A' atoms at the face centers]
The number of 'B' atoms = (12 x 1/4) + (1 x 1) = 3 + 1 = 4 [there are 12 'B' atoms at the edges and 1 'B' atom at the center of the unit cell]
However after removing two face centered 'A' atoms along one of the axes (x axis) as shown below :
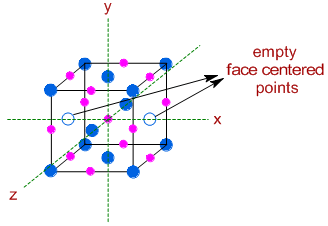
The number of 'A' atoms = (8 x 1/8) + (4 x 1/2) = 1 + 2 = 3 [there are now only 4 'A' atoms at the face centers]
The number of 'B' atoms = (12 x 1/4) + (1 x 1) = 3 + 1 = 4
Therefore the stoichiometry after removing these atoms is: A3B4
Note:
Each atom at the corner contributes 1/8th, and each atom at the face center contributes 1/2nd and each atom along the edge contributes 1/4th to the unit cell.
Conclusion:
The correct option is '4'.
(IIT JEE 2002)
A) AB3
B) A4B3
C) A3B
D) Composition cannot be specified.
Solution:
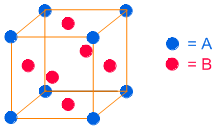
The 'A' atoms occupy 8 corners and their number = 8 x 1/8 = 1
The 'B' atoms occupy 6 face centers and hence their number = 6 x 1/2 = 3
Conclusion:
The composition of AxBy is AB3.
(KURUKSHETRA CEET 1996)
a) Na2WO3
b) Na2WO2
c) NaWO2
d) NaWO3
Solution:
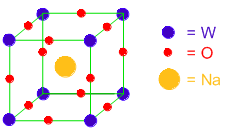
Number of 'W' atoms = 8 x 1/8 = 1 (since these are at the corners)
Number of 'O' atoms = 12 x 1/4 = 3 (because 'O' atoms are at the centers of face edges)
Number of 'Na' atoms = 1 x 1 = 1 (The Na atom is at the center of the cube)
Conclusion:
The formula of the compound is NaWO3.
The value of number of anions to cations in Z is:
(IIT JEE ADVANCED 2018 solved chemistry question)
Solution:
Watch the following video for animated solution.
1) What is FCC lattice?
2) What is unit cell?
3) If all the ions about one of the C3 axis of symmetry in NaCl crystal are removed, the new formula of the compound is ........? (Na12Cl15)
Hint: C3 axis of symmetry passes through the body diagonal of the cubic unit cell of NaCl crystal. Along this axis, there are two Cl- ions at the corners and one Na+ ion exactly at the center of cubic cell.
4) In an alloy, atom 'X' occupies the corner positions and atom 'Y' occupies the face center positions of FCC lattice. The formula of the compound is.....? (XY3)
5) A solid has a structure in which ‘W’ atoms are located at the corner of the unit cell, ‘O’ atoms are located at the cube edge and ‘Na’ atoms at the cube center. The formula of the compound is..... (NaWO3)
6) Three atoms A, B and C crystallize in a cubic solid lattice where A atoms are present at the body centre, B atoms are present at the edge centres as well as at the corners of the cube and C atoms are present at the face centres of the cube. Now if all the atoms are removed from the two 4-fold axes and the one 2-fold axis passing through the cube, then the formula of the compound is........ (B7C2)
7) In a crystal of NaCl, if all the ions along one of the body diagonal are removed, then the simplest formula of the crystalline solid will be........... (Answer: Na12Cl15 or Na15Cl12)
8) In a solid AB having rock salt structure, if all the atoms touching 1 body diagonal plane are removed plane are removed (except at body centre), then the formula for the left unit cell is........... (Answer: Na16Cl15 or Na15Cl16)
9) If all the ions from (i) one of the rectangular planes passing through opposite face centres as well as (ii) one of the triad axis of symmetry are removed, then which on eof the following statements is incorrect? (ignore charge balance)
A) Formula of NaCl becomes Na8Cl7
B) Formula of Na2O becomes Na24O7
C) Formula of ZnS becomes Zn3S7
D) Formula of CaF2 becomes Ca7F24
10) Three atoms a, b and c crystallize in a cubic solid lattice where a atoms are present at the body centre, b atoms are present at the edge centre as well as at the corners of the cube and c atoms are present at the face centres of the cube. Now if all the atoms are removed from the two 4-fold axis and the one 2-fold axis passing through the cube, then the formula of the compound is.........
11) In an alloy atom x occupies the corner positions and atom y occupies the face centre positions of fcc lattice. If one of the face centre atom is missing then formula of the compound is.........