Pericyclic reactions are the concerted reactions involving reorganization of electrons which occur by the way of a single cyclic transition state.
Characteristics of Pericyclic reactions:
* The pericyclic reactions occur in single step and hence there is no intermediate formed during the reaction.
* The breaking and making of bonds (both σ & π) occur simultaneously in a cyclic transition state.
* The configuration of the product depends on
1) the configuration of reactants
2) the number of electron pairs undergoing reorganization and
3) the reaction conditions (like thermal or photochemical).
The pericyclic reactions are further classified into following types:
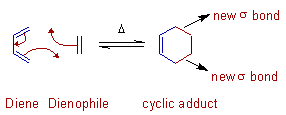
Cycloaddition reactions involve the formation of a cyclic product due to addition of two different π bond containing components, which are joined by newly formed two σ bonds at their ends at the expense of two π bonds.
It is usually reversible and the backward reaction is also referred to as retro-cycloaddition or a cycloreversion.
The classic example of cycloaddition is Diels-Alder reaction between a Diene and a Dienophile to give a cyclic adduct.

Note: The direction of curly arrows has no significance here. The movement of electrons can be shown either clockwise or anti clockwise.
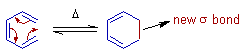
Electrocyclic reactions are intramolecular pericyclic reactions which involve the rearrangement of π-electrons in an open conjugated system leading to formation of a cyclic product with a new σ bond at the expense of a π-bond.
However the electrocyclic reactions not only involve ring-closure but also ring opening, which are referred to as retro-electrocyclic reactions.
E.g. The formation of Cyclohexa-1,3-diene by heating Hexa-1,3,5-triene is an example of ring-closure electrocyclic reaction.
Sigmatropic rearrangements are concerted unimolecular isomerization reactions characterized by the overall movement of a σ-bond from one position to another with an accompanying rearrangement of π-electrons of conjugated system so as to accommodate the new σ-bond.
E.g. The [3,3] Cope rearrangement. The σ-bond undergoing movement is shown as red thick line.
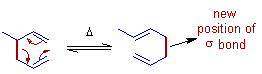
Note: Though looking like electrocyclic reactions, there is no reduction in the number of π-bonds in sigmatropic reactions.
The concerted transfer of a group from one molecule to another due to concomitant movement of a σ-bond (from one molecule to another) and formation of a new σ-bond (between two molecules) at the expense of a π-bond is generally referred to as group transfer pericyclic reaction.
E.g. The Ene reaction between propene and ethene to give 1-pentene is a classic example of group transfer reaction.
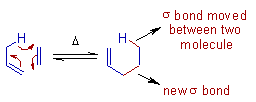
These reactions resemble sigmatropic rearrangements, since a σ-bond moves. However sigmatropic reactions are unimolecular reactions whereas the group transfer reactions are bimolecular.
They also resemble cycloadditions, since a new σ-bond is formed at the expense of a π-bond. However, in group transfer reactions, no ring is formed.
Cheletropic reactions are a special class of cycloadditions or retro-cycloadditions in which the two σ-bonds are either made or broken to the same atom.
E.g. The reversible addition of sulfur dioxide to 1,3-butadiene is an example of cheletropic reaction, in which the two new σ-bonds (shown in red) are made to the sulfur atom.
![]()
Note: In this reaction, a lone pair on sulfur atom is equivalent a π-bond and is reorganized. One π-bond and a lone pair are disappeared, whereas two σ-bonds are formed.
Also note that sulfur atom is oxidized from +4 to +6 state.
The pericyclic reactions which involve concerted intramolecular migration of two σ-bonds simultaneously are known as dyotropic rearrangements.
However dyotropic reactions can also occur stepwise.
There are two types of dyotropic rearrangements:
Type-I: Two migrating groups interchange their relative positions

Type-II: The σ-bonds are migrated to new bonding sites without any positional interchange for groups.

All types of pericyclic reactions are concerted and involve cyclic transition state without any intermediate formed during the reaction. The characteristics which differentiate them from each other are tabulated below.
| S.no | Type of pericyclic reaction | change in
no. of σ bonds |
change in
no. of π bonds |
comments |
| 1) | Cycloaddition reactions | +2 | -2 | A cyclic product is formed; may be intermolecular or intramolecular. |
| 2) | Electrocyclic reactions | +1 | -1 | Intramolecular. |
| 3) | Sigmatropic reactions | 0 | 0 | Intramolecular; migration of a σ-bond; rearrangement of π-electrons |
| 4) | Group transfer reactions | +1 | -1 | Intermolecular transfer of a group; migration of a σ-bond from one molecule to another; formation of new σ-bond at the expense of one π-bond. |
| 5) | Cheletropic reactions | +2 | -1 (π-bond) -1 (lone pair) |
A cyclic product is formed; two σ-bonds are formed to same atom; A lone pair is disappeared. |
| 6) | Dyotropic reactions | 0 | 0 | Simultaneous migration of two σ-bonds. |
It is observed that some of the pericyclic reactions occur only upon heating, whereas the other are possible only under photochemical conditions.
E.g. The Diels-Alder reaction, a [4+2] cycloaddition occurs under thermochemical conditions and is not possible under photochemical conditions.
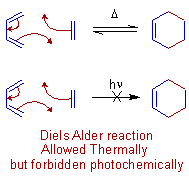
Whereas the following [2+2] cycloaddition is forbidden under thermal conditions. But the reaction is possible under photochemical conditions.
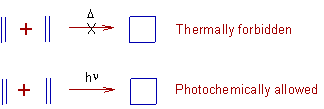
Even though, most of the times, the final products are thermodynamically stable, there is a high kinetic barrier due to symmetry considerations under particular conditions to make the reaction forbidden. However the same symmetry considerations allowed the reaction in different conditions.
Frontier Molecular Orbital (FMO) theory proposed by Kenichi Fukui in 1952, explains whether a pericyclic reaction is allowed or not under given set of reactions conditions based on interactions between frontier molecular orbitals (FMOs) like HOMO, LUMO & SOMO.
HOMO = Highly Occupied Molecular Orbital
LUMO = Lowest Unoccupied Molecular Orbital
SOMO = Singly Occupied Molecular Orbital
The interaction between one FMO of one molecule with one FMO of another molecule results in two types of new Molecular Orbitals (MOs) i.e., bonding and antibonding. The bonding orbitals possess low energy, whereas the antibonding orbitals possess higher energy.
If both of these resulting MOs are filled with electrons, the bonding interaction is cancelled by the anti bonding interaction. Hence the net result is no bonding between molecules.
However, if only bonding orbitals are filled with electrons, the two molecules attract with each other.
* Interaction between HOMO & HOMO causes repulsion i.e., no bonding interaction since both bonding and antibonding MOs are filled with electrons.
* Interaction between HOMO & LUMO causes attraction i.e., bonding interaction, since only the bonding MO is filled with electrons.
* Interaction between LUMO & LUMO causes neither attraction nor repulsion since all the resulting MOs are empty.
* Interaction of SOMO with either HOMO or LUMO or another SOMO also causes attraction between the interacting species.
The effects of interactions between frontier molecular orbitals is summarized in the following table.
| Interacting Frontier Molecular Orbitals |
Type of Interaction |
| HOMO + HOMO | No bonding |
| HOMO + LUMO | Attraction - Bonding |
| LUMO + LUMO | No electrons, null interaction - No bonding |
| SOMO + HOMO SOMO + LUMO SOMO + SOMO |
Attraction - Bonding |
To predict whether a pericyclic reaction is allowed or not under given condition, Woodward and Hoffmann proposed following set of rules based on conservation of orbital symmetry concept.
A thermal pericyclic reaction is allowed in the ground state, when the total number of (4q + 2)s and (4r)a components is odd.
Otherwise, if the total of (4q + 2)s and (4r)a components is even, the pericyclic reaction is allowed in the excited state i.e., under photochemical conditions.
| Number of (4q + 2)s and (4r)a components | The condition under which the reaction is allowed |
| odd | Thermal |
| even | Photochemical |
Component: A bond(s) or an orbital(s) taking part in the pericyclic reaction as a single unit can be considered as a component. It can have any number of electrons but may not have mixtures of π and σ electrons.
E.g.
A double bond is considered as a π2 component, since there are two π electrons.
A conjugated diene can be considered as π4 component, since there are four π electrons.
's' represents suprafacial. A suprafacial component forms new bonds on the same face at its both ends. In some cases suprafacial is equivalent to "dis-rotation".
'a' represents antarafacial. An antarafacial component forms new bonds on the opposite faces of its both ends. In some cases antarafacial is equivalent to "con-rotation".
E.g.
π2s represents a component containing two π electrons and forming new bonds in suprafacial manner.
π4a represents a component containing four π electrons and is going to form new bonds in antarafacial manner.
q & r: These are integers.
(4q + 2)s component: The suprafacial component, which may have either 2 or 6 or 10 or _ _ _ electrons of same type. These numbers are obtained by substituting 'q' by 0 or 1 or 2 or _ _ _.
(4r)a component: The antarafacial component, which may have either 4 or 8 or 12 or _ _ _ electrons of same type. These numbers are obtained by substituting 'r' by 1 or 2 or 3 or _ _ _.
Likewise the meanings of (4q + 2)a & (4r)s can be understood.
Application:
Let us assume the diene and dienophile in Diels-Alder reaction are approaching suprafacially as shown below.
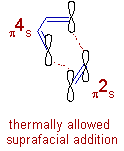
Since there are 4 π electrons in diene, which is making bonds in suprafacial manner, it is a (4r)s component.
And the alkene is a (4q + 2)s component, since it has 2 π electrons and is approaching the diene suprafacially.
i.e, there is one (4q + 2)s component and there are no (4r)a components.
Hence, the total number of (4q + 2)s and (4r)a components = 1 + 0 = 1, an odd number.
Therefore Diels-Alder reaction is thermally allowed in ground state when both the components are approaching suprafacially. Hence it is termed as π4s + π2s cycloaddition.
Antarafacial addition, for this reaction, is not allowed under thermal conditions. But it is theoretically allowed under photochemical conditions in the excited state. However, the strain in the transition state while doing so forbids to do so.
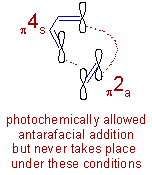
Note: The orbitals shown in above diagrams are simple 'p' orbitals and are not the frontier molecular orbitals. Do not mix descriptions of FMO theory with Woodward-Hoffmann rules.
Since application of above Woodward-Hoffmann rules to pericyclic reactions is tedious and cumbersome, the following simplified rules based on aromatic transition state proposed by Zimmerman can be used to predict theoretically allowed modes of pericyclic reactions under given conditions.
These rules are based on the concept of topology of aromatic transition state. The cyclic transition state with 4n+2π electrons has Huckel topology under thermal conditions and Mobius topology under photochemical conditions. Hence supra facial interaction between orbitals is allowed under thermal conditions, whereas antara facial interaction is allowed under photochemical conditions.
Whereas, the cyclic transition state with 4nπ electrons has Mobius topology under thermal conditions and Huckel topology under photochemical conditions. Hence antara facial interaction between orbitals is allowed under thermal conditions, whereas supra facial interaction is allowed under photochemical conditions.
| No. of π electrons | Reaction conditions | Type of
Aromaticity in Transition state |
Allowed mode |
| (4n+2) A Huckel number |
Thermal | Huckel | Supra (or) Dis |
| Photochemical | Mobius | Antara (or) Con | |
| (4n) A non Huckel number |
Thermal | Mobius | Antara (or) Con |
| Photochemical | Huckel | Supra (or) Dis |
Remember that even though the pericyclic reactions are allowed theoretically under both the conditions, most of the times the factors like steric hindrance and strain in the transition state may forbid the reaction in particular mode, especially the antara facial.
The detailed usage of these rules and theoretical base are explained in the later sections.