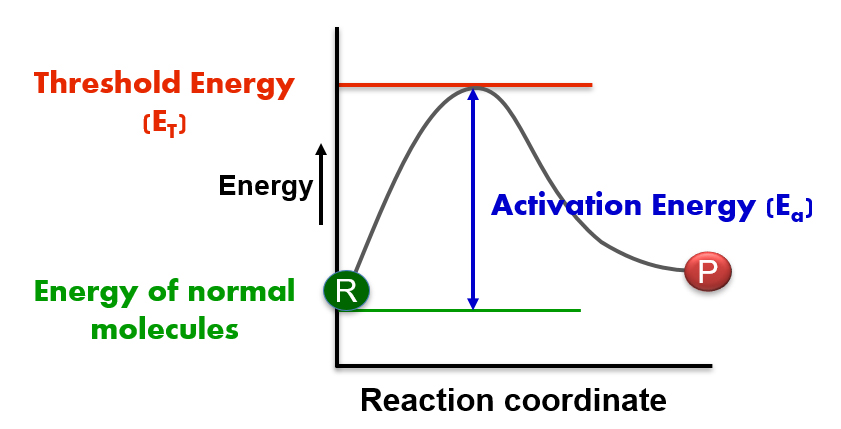
Activation energy (Ea) is an important concept in chemistry that describes the amount of energy that should be acquired by the normal reactant molecules to cross the threshold energy barrier thereby initiating a specific chemical reaction through a specific mechanistic path. Following is the exact definition of activation energy.
The difference between threshold energy (ET) and average energy (ER) of the reacting molecules is called Activation energy (Ea).
Ea = Threshold energy (ET) - Average energy (ER)
The minimum amount of energy required by the colliding molecules to yield the products is called Threshold Energy (ET).
The reactant molecules must cross this energy barrier to be converted to products.
The energy possessed by the normal reactant molecules under STP conditions is called 'Average Energy' .
Note: Since 1982, according to IUPAC, the Standard Conditions refer to 0oC and 100 kPa (1bar).

The rate of a chemical reaction is intricately tied to the concept of activation energy. This vital parameter exhibits an inverse relationship with the speed of the reaction. The higher the activation energy, the slower the reaction will proceed, as it requires more energy for the reactant molecules to overcome the barrier and transform into products. On the other hand, a lower activation energy means that the reaction can occur more quickly, as less energy is needed for the molecules to reach the necessary threshold energy barrier.
The activation energy (Ea) is related to the rate constant (k) of a reaction at temperature, T is given as:
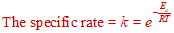
Where:
k = rate constant
Ea = Activation Energy
R = Universtal gas constant
T = Absolute temperature in kelvins
At two different temperatures, the equation can be written as follows.
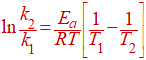
Where,
k1= rate constant at temperate T1
k2= rate constant at temperate T2
In practical terms, understanding activation energy allows chemists to optimize reaction conditions and design catalysts that can lower the activation energy, making reactions more efficient and cost-effective. By quantifying the energy barrier that reactant molecules must overcome to transform into products, scientists can elucidate reaction mechanisms, optimize reaction conditions, and design efficient catalysts.
Question-1) Choose the correct statement about activation energy
A) Activation energy is the amount of energy possessed by the reactant molecules to undergo a chemical reaction
B) Activation energy is different at different temperatures for a particular chemical reaction.
C) The rate of reaction increases with increase in the activation energy.
D) None of the above
Answer: D
Explanation:
Activation energy is the energy required by the normal molecules under standard condition to surpass the threshold energy barrier of the reaction. It is inversely proportional to the rate of a chemical reaction and does not change with temperature by definition.
Question-2) Given that the activation energy of an endothermic reaction is 150 kJ/mol, which of the following statements are correct?
A) The reverse reaction has an activation energy equal to 150 kJ/mol.
B) The reverse reaction has an activation energy less than 150 kJ/mol.
C) The reverse reaction has an activation energy greater than 150 kJ/mol.
D) All are incorrect.
Answer: B
Explanation:
In endothermic reactions, the products are associated with more energy and hence require less energy to cross the same energy barrier while converting back to reactants.