Explanation:
Ethyl chloride can undergo both nucleophilic substitution as well as elimination reactions with strong alkali like KOH.
When it undergoes nucleophilic substitution, ethyl alcohol is formed as major product.
![]()
Mechanism of nucleophilic substitution:
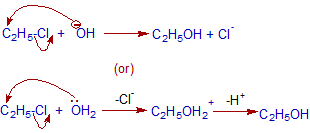
Above mechanism depicts SN2 path. Usually primary alkyl halides show Nucleophilic bimolecular substitutions since the formation of carbocation is less easy. Hence bond breaking and making occurs in one step.
However, due to presence of β-hydrogen, ethyl chloride can also undergo elimination reaction to give ethylene in presence of strong base like KOH.
![]()
Since KOH is a strong base, it can also abstract a β-hydrogen and thus by favoring elimination of HCl molecule (dehydrohalogenation).
Mechanism of dehydrohalogenation of ethyl chloride:
The elimination of hydrogen halide may occur by E1 or E2 mechanism.
E1 path
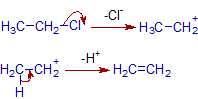
E2 path
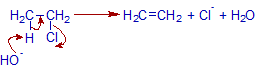
Since ethyl chloride is a primary alcohol, the preferred path is E2.
Answer:
Now we can safely assume that:
i) When water is used as solvent, the likeliness of nucleophilic attack by H2O as well as OH- is greater. That leads to formation of nucleophilic substituted product.
ii) In aqueous solutions, the elimination is not favored due to formation of water as one of the product. It is according to le Chatelier’s principle. Excess of water will shift the equilibrium to the left side.
iii) When the reaction is carried out in alcoholic KOH, less number of water molecules are available and thus by increasing the chances of attack of OH- ion on β-hydrogen.
iv) Moreover, in alcoholic KOH, the solvent used is ethyl alcohol which is incidentally the nucleophilic substitution product. According to le Chatelier's principle, this pathway must be less favored as the container is full of ethyl alcohol that results in shifting of equilibrium (involving substitution) to the left side. Meanwhile, the competing elimination mechanism operates and thus by producing ethylene which escapes out as a gas and shifting equilibriium (involving elimination) to the right side.
1) Which gas can be produced by the dehydrohalogenation of ethyl chloride?
Answer: Ethylene (ethene) gas is produced when ethyl chloride (chloroethane) is dehydrohalogenated with strong bases like alcoholic KOH.
CH3CH2Cl + alc. KOH ------------>CH2=CH2 + KCl + H2O
2) Why tertiary alkyl halides undergo elimination reactions in presence of strong bases rather than nucleophilic substitution?
3) Why primary alkyl halides take SN2 or E2 paths?
4) Write rate expressions for SN1 and SN2 mechanisms.
5) What is alcoholic KOH?
Answer: It is KOH dissolved in alcohol like ethylalcohol.
6) Which gas is produced on dehydrohalogenation of ethyl iodide (Iodoethane) - CH3CH2I?
Answer: Ethylene
Author: Aditya vardhan Vutturi