Energy is required to overcome the attraction between nucleus and the electron while removing it from the atom. This energy is referred to as ionization energy and is expressed in kJ mol-1. It is a measure of nuclear attraction over the outermost electron in a given species (atom or ion or molecule).
Previously it was denoted by ionization potential expressed in eV atom-1. Physicists still follow this convention.
The ionization of an atom or an ion or a molecule (by removing electron) results in a positively charged species and is always an endothermic reaction i.e. energy is absorbed. Therefore, the enthalpy of ionization is a positive quantity.
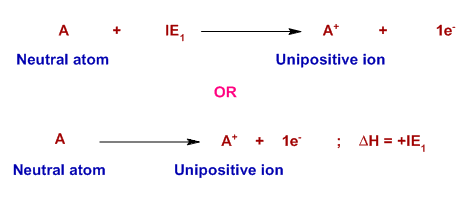
The first ionization energy is defined as the minimum amount of energy required to remove one electron from the outermost orbit of gaseous neutral atom in its ground state. It is represented by IE1.

Note: There are two ways to represent a thermochemical reaction. In the first one, the ionization energy is indicated on the left side of the reaction since it is added to the atom. It is an endothermic reaction.
However, in the second representation, the ionization energy is represented as enthalpy of reaction, ΔH, which is the difference in the enthalpies of products and reactants (Hproducts- ΔHreactants). It is positive since ionization is an endothermic reaction.
The second ionization energy is the minimum amount of energy required to remove an electron from the outermost orbit of gaseous unipositive ion in its ground state. It is represented by IE2.
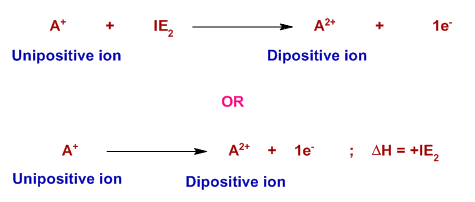
The second ionization energy is always greater than the first as the effective nuclear charge increases from A to A+.
In general the order of ionization energies can be written as:
IE1 < IE2 < IE3 < IE4 _ _ _ _ _
One should keep in mind that the energy required to remove second electron is greater than that of the first electron. Likewise, removal of third electron is difficult than the removal of second one.
It is easy to recognize that the force of attraction between nucleus and electron decreases with increase in the distance between them. As a consequence, the ionization energy decreases with increase in atomic radius since the nuclear attraction over outer electron decreases. Thus farther the electron from nucleus, easier will be the removal.
E.g., Down the group, the ionization energies decrease with increase in atomic radii.
Order of ionization energies in IA group -------> Li > Na > K > Rb > Cs > Fr
Ionization energy increases with increase in the effective nuclear charge. It is evident from the trend observed in first and second ionization potentials.
Effective nuclear charge refers to the nuclear attraction experienced by the electron. It is proportional to the number of protons per electron (i.e. ratio of protons to electrons).
Note: There are other factors affecting this.
For example, when an electron is removed from a neutral atom, the number of protons attracting the remaining electrons in the ion formed increases. Hence the second ionization energy is always greater than the first one.
Reduction in nuclear attraction over the outer electrons due to repulsions by inner electrons is called screening or shielding effect.
Ionization energy decreases with increase in screening power of inner electrons.
The order of screening power of electrons present in different types of orbitals is: s > p > d > f .
The ability of an electron to come closer towards nucleus is called penetration power. Electrons will have the natural tendency to come closer towards the nucleus. However, the time they spend closer to the nucleus depends on the shape of orbital as well as the principal quantum number.
Greater the penetration power, greater will be the ionization energy.
The order of penetration power of electrons in different orbitals is as follows.
s > p > d > f
Atoms with stable electronic configurations like: ns2 np6 or half filled or completely filled sub shells are extra stable. Removal of electrons from them is difficult and hence their ionization energies are comparatively high.
These configurations are more symmetric and the exchange energy is minimal. Hence they are relatively more stable.
E.g. Noble gases with ns2 np6 ; IIA group elements with ns2 (completely filled); and VA group elements with ns2 np3 (half filled) configurations have higher ionization potentials.
Ionization energy decreases down the group as the atomic radius increases and effective nuclear charge decreases. The effective nuclear attraction decreases due to screening by the inner electrons.
E.g. The order of ionization energy in IA group elements is Li > Na >K > Rb >Cs > Fr
In general, the ionization energy increases from left to right in a period with decrease in atomic radius and increase in effective nuclear charge. However, the trend is not regular. There are dips in the ionization energies of elements of group 13 and group 16.
In a given period, group 13 (IIIA group) elements with ns2np1 configuration have lower ionization energies than group 2 (IIA group) elements with ns2 configuration. It is due to easy removal of p-electron with less penetration power than that of s-electrons.
Note: It is some times wrongly reasoned as due to extra stability of full filled s-sublevel, which is misleading.
The elements of group 16 (VI A) with ns2np4 configuration possess smaller first ionization energy values than elements of group 15 (V A) with ns2np3 configuration. It is due to repulsion experienced by the differentiating electron (last one) that must enter into one of the half filled p-orbital, resulting in a lower ionization energy.
E.g., The order of ionization energies in 2nd period is given below:
| Li < | Be > | B < | C < | N > | O < | F << | Ne |
| 2s2 | 2s22p1 | 2s22p3 | 2s22p4 | 2s22p6 | |||
| greater penetration power of s-electron | easy removal of p-electron | half filled p-sublevel & hence no repulsions between p-electrons | repulsion between paired p-electrons | highly stable octet configuration |
In a given period alkali metals have very low first ionization energies (IE1) and noble gases have very high IE1 values.
The helium atom has highest ionization energy of any atom whereas Cesium has the lowest in the periodic table.
In d-block elements, increase in the 1st and 2nd ionization energies across a period is not much considerable since they involve removal of s-electrons, which are shielded from the increasing nuclear charge by inner d-electrons.
It results in reduction of the rate at which effective nuclear charge, Zeff for a 4s electron increases with atomic number, Z. Hence 1st and 2nd IE's are relatively independent of atomic number, Z and the IE curves are not much steeper.
However, there is considerable increasing trend observed in case of 3rd ionization energies since 3rd IE involves removal of electron from inner d-orbitals. which do not shield one another efficiently from the nuclear charge. As a result, the 3rd IE’s are more sensitive to increasing in Z.
In case of 1st row transition elements, there is discontinuity in the increasing trend of 3rd IE between Manganese and Iron (i.e. 3rd IE of Mn > 3rd IE of Fe). This is because of additional energy required to remove electrons from Mn2+ with stable half filled d-sublevel. The extra stability is due to exchange energy associated with half-filled sub levels. These systems contain electrons with parallel spins with smaller electron-electron repulsions. They tend to occupy larger volume and much more stabilized than the electron systems with anti parallel spins.
The same trend is observed in 3rd ionization energies of 2nd row transition elements between Technitium and Ruthenium (i.e. 3rd IE of Tc > 3rd IE of Ru).
In f-block elements (both lanthanides & actinides), an insignificant change in the first & second ionization energy values is observed with increase in the atomic number due to shielding effect of f-electrons. Thus the first and second IEs of f-block elements are less sensitive to increasing atomic number.
However, as in case of d-block elements, the increasing trend in 3rd ionization energies is more steeper but is far less regular.
The half-filled 4f7 and full-filled 4f14 sub shells are more stable due to exchange energy. These systems are associated with greater 3rd ionization energies.
For example, in lanthanides, the third IE increases strongly to Eu2+, where the 4f7 configuration is reached. Thereafter it falls back and then increases again on approaching the 4f14 configuration at Yb2+.
The trend in 3rd ionization energies of actinides is less simple than for lanthanides due to relativistic effects.