We know that matter is anything that contains mass and occupies space. Depending on how it occupies space; relative kinetic energies and the magnitude of forces of attraction between constituent particles, there are four distinct states of matter i.e.,
These states are inter-convertible. The conversion is usually brought about by changing the temperature or pressure or both.
Note: The constituent particles may be atoms or molecules or ions.
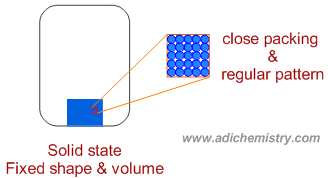
* In the solid state, matter occupies fixed volume and has fixed shape.

* The forces of attraction between particles are very strong such that the constituent particles are very closely arranged, most often in a regular pattern with fixed spatial geometry.
* In this state the kinetic energies of particles are very low so that they cannot escape from the attractions of surrounding particles. They have only the freedom of vibration and little freedom to rotate and translate their positions.
* Usually, the density of a substance is very high in solid state than other states. However there are exceptions like water.
* Solid are practically incompressible since the particles are closely packed.
* The liquid state has fixed volume but has no definite shape. It adapts to the shape of container. Indeed, it tends to occupy the bottom portion of the container due to gravitational pull and has free surface at the top.
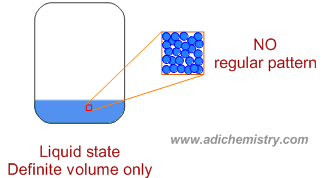
* The forces of attraction are less when compared to that of solid state.
* In the liquid state, the kinetic energies more than in solid state such that the constituent particles are free to translate their positions and do not have long range orders (fixed spatial geometry). However still they maintain the proximity with each other.
They can slide past each other and cannot go away from each other.
* In general, the density of liquid state of a substance is less than that of solid state.
* Liquids are slightly compressible.
* The gaseous state of matter has neither definite volume nor definite shape. It occupies the entire volume of the container in which it is filled and takes the shape of the container.
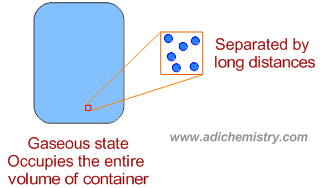
* The forces of attractions between the constituent particles are negligible due very high kinetic energies, which are more than sufficient to escape from the attractions of other particles and tend to maintain very long distances from each other.
* The particles in the gas move randomly in all directions with very high velocities and tend to deny the gravitation force of attraction.
* The actual volume of the constituent particles is negligible when compared to the volume of the container.
Note:
* Since the gas molecules move with high velocities, they collide with the walls of the container with some force. We know that force per unit area is called pressure.
* Another term we use for gaseous state of a liquid is "vapor". The pressure exerted by vapor is called vapor pressure.
* The term used for hot vapors of a liquid is "steam", especially escaping out from an outlet with certain force.
* Plasma state is the fourth state of matter, which consists of ionized gaseous atoms or molecules along with electrons. This state is obtained by removing electrons from the atoms or molecules by heating gases at very high temperatures.
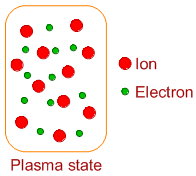
* It is estimated that about 99% of universe is in the plasma state. The examples include charged air produced during lightening, the matter in sun and stars, the state of gas inside neon light or fluorescent light etc.,
However plasma state if very rare in the atmosphere of earth. We are surrounded by materials which are mostly solids or liquids or gases and not plasma.
It is possible to interconvert above states by changing temperature or pressure or both.
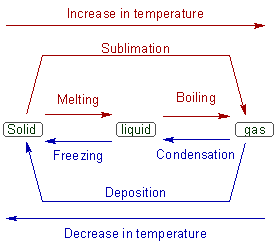
Melting: The conversion of solid to liquid is referred to as melting or fusion. It is achieved by heating the solid.
Freezing: The reverse of melting is called freezing. The liquid is converted to solid in this process by lowering the temperature.
Boiling: Conversion of liquid into vapour or gas is known as boiling. It is achieved by increasing the temperature of liquid.
Condensation: It is the reverse of boiling. The gas is converted to liquid by lowering the temperature.
Sublimation: Sometimes, solids are directly converted into vapour upon heating. This process is knows as sublimation.
Deposition: This is reverse of sublimation. The vapors are directly converted to solid.