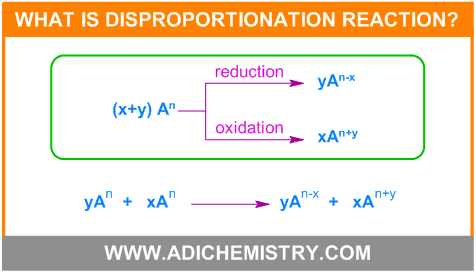
What is disproportionation reaction?
The redox reaction that involves simultaneous oxidation and reduction of atoms of same element from one oxidation state (OS) to two different oxidation states is known as disproportionation reaction.

Note: The disproportionation reaction is also known as dismutation reaction.
The requirement for disproportionation reaction to occur is, the element undergoing disproportionation should exhibit minimum three different oxidation states and the element must be less stable in a particular oxidation state from which it can be both oxidized as well as reduced to relatively more stable oxidation states.
When three oxidation states of an element are written in the increasing (or decreasing) order, the element in the middle oxidation state should be less stable and the higher and lower oxidation states have to be relatively more stable as well as the overall process must be thermodynamically feasible.
The Latimer and Frost diagrams are used to predict whether an atom in particular oxidation state undergoes disproportionation or not (thermodynamic feasibility).
In the Latimer diagram, the unstable species that can undergo disproportionation has a more positive emf value to its right (for reduction) than the emf to its left (for oxidation).
It is also possible to get the same information from Frost diagrams. The Unstable chemical species will be found at higher point on the plot than the line connecting their neighbors.
Dissociation of hydrogen peroxide is a disproportionation reaction. The oxygen atom in H2O2 is in -1 oxidation state. It is oxidized to O2 (ox.st = 0) as well as reduced to H2O (ox.st = -2).
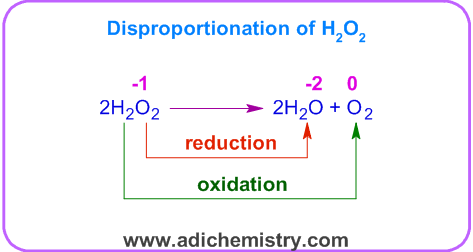
In above redox reaction, the relatively less stable peroxide disproportionate into relatively more stable compounds i.e. water and dioxygen.
Except fluorine, halogens (Cl2 ,Br2, I2) undergo disproportionation in alkaline medium.
They form halide and hypohalite in cold and dilute alkali. For example, chlorine disproportionates to sodium chloride and sodiumchlorite when treated with cold and dilute sodium hydroxide solution.
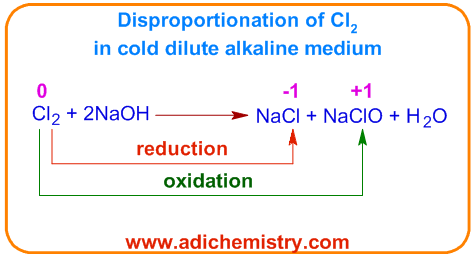
In above disproportionation reaction, one chlorine atom is oxidized to +1 while the second one is reduced to -1 oxidation states.
Whereas, halide and halate ions are formed in hot and concentrated alkali. For example, as shown below, when chlorine gas is passed through a hot and concentrated solution of caustic soda, it is reduced to chloride ion and oxidized to chlorate ion.
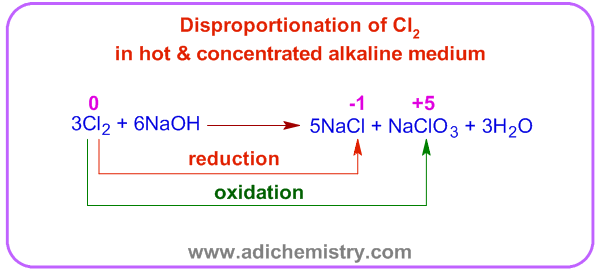
In this case, one chlorine atom loses 5 electrons and oxidized to +5 state while the five chlorine atoms are reduced to -1 state by accepting one electron each.
Bromine and Iodine also show the same behaviour in alkaline solutions.
However, fluorine does not show disproportionation reactions as it can exist only in two oxidation states i.e., 0 and -1 due to its high electronegativity and very high ionization energies. It cannot be oxidized by any other element. Indeed, its most stable oxidation state is -1 and cannot be easily oxidized to zero oxidation state chemically.
Phosphorus disproportionates to phosphine and hypophosphite in alkaline medium. In this case, one P atom is reduced to -3 oxidation number (in PH3) and three P atoms get oxidized to +1 (in NaH2PO2).
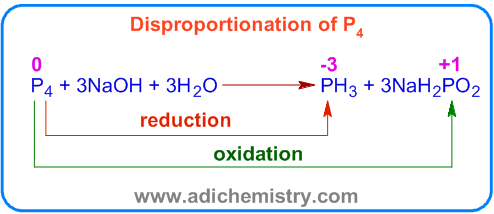
Mercurous chloride undergoes disproportionation under UV light to give mercury and mercuric chloride. The Hg22+ ion is oxidized to Hg2+ and reduced to Hg.
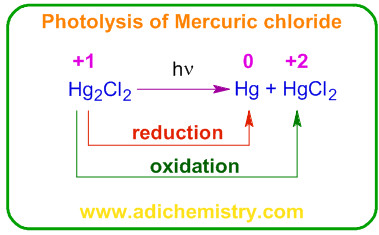
Nitrogen dioxide, NO2 reacts with water to give nitric and nitrous acids (Ostwald process). It is a disproportionation reaction. The Nitrogen in NO2 is in +4 oxidation state. It is reduced to nitrous acid, HNO2 in which the OS of nitrogen is +3 and oxidized to nitric acid, HNO3 in which the OS of nitrogen is +5.
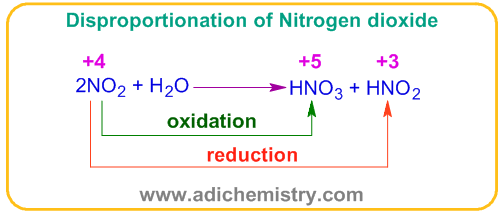
The interhalogen compounds, especially of XY type, tend to undergo disproportionation reaction. For example, Brominefluoride, BrF disproportionate to Brominetrifluoride, BrF3 and bromine.
In above dismutation reaction, bromine is oxidized to +3 (as in BrF3) and reduced to 0 oxidation states (as in Br2) from +1 oxidation state as in BrF.
Based on ease of disproportionation, the stability of XY type inter-halogen compounds is in the order: ClF>ICl>IBr>BrCl>BrF.
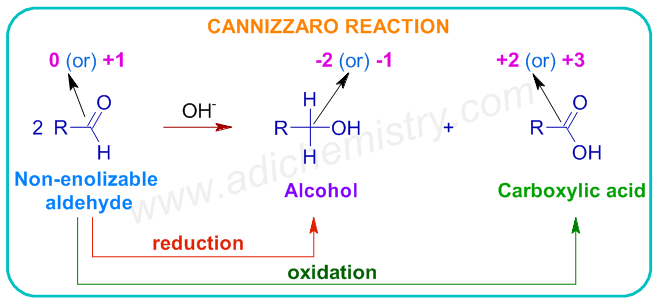
The Cannizzaro reaction is an example from organic chemistry for disproportionation reaction. In this reaction, the aldehydes, without α-hydrogens, in presence of a strong base, disproportionate to furnish an alcohol and a carboxylic acid. One molecule of aldehyde is reduced to the corresponding alcohol, while a second one is oxidized to the carboxylic acid.
1) Which one of the following is an example of a disproportionation reaction?
1) 4H3PO3 → 3H3PO4 + PH3
2) 2NO2 + H2O → HNO3 + HNO2
3) HgCl2 → Hg + HgCl2
4) All
Answer: 4
2) Which one of the following is NOT an example of a disproportionation reaction?
A) Cl2 + 2NaOH → NaCl + NaOCl + H2O
B) P4+ 3NaOH + 3H2O = PH3 + 3NaH2PO2
C) 2NaOH + 2F2 → 2NaF + OF2 + H2O
D) 2H2O2 → 2H2O + O2
Answer: C
Explanation: When fluorine is bubbled through cold and dilute sodium hydroxide, it oxidizes the O2- to O2+ (in OF2).
3) Which following element does not show the tendency to undergo disproportionation reaction?
A) oxygen
B) chlorine
C) fluorine
D) Manganese
Answer: C
Explanation: Fluorine does not undergo disproportionation reaction since it exhibits only two oxidation states i.e. 0 and -1.
Related question: Why fluorine does not show disproportionation reactions?
4) Identify disproportionation reaction. (Chemistry MCQ IIT JEE NEET)
A) 2NO2 + 2OH– → NO2 – + NO3 – + H2O
B) CH4 + 4Cl2 → CCl4 + 4HCl
C) CH4 + 2O2 → CO2 + 2H2O
D) 2NaOH + 2F2 → 2NaF + OF2 + H2O
Answer: A
5) Which of the following ion of manganese undergoes disproportionation reaction?
A) Mn2+
B) MnO42-
C) MnO4-
D) None
Answer: B
Explantion: To undergo disproportionation from one particular oxidation state, there should be one higher oxdiation state and one lower oxidations state than it. Manganese can show +2 to +7 oxidation states along with zero oxidation state. Since Mn in MnO42- is already in the higher oxidation level i.e. +7, it cannot undergo disproportionation.
Mn2+ ion is stable and cannot disproportionate to zero oxidation state (lower) and any of the higher oxidation state (from +3 to +7) as the reaction is not thermodynamically feasible.
However, MnO42- ion in which the Mn is in +6 oxidation state can disproportionate to +7 and +2 oxidation states and the reaction is also thermodynamically feasible.
Related question: MnO42- undergoes disproportionation reaction in acidic medium but MnO4- does not. Give reason.
6) Disproportionation means (MCQ)
A) A redox reaction in which atoms of an element from one oxidation state are simultaneously oxidized as well as reduced.
B) A redox reaction that involves oxidation of atoms of an element to two different oxidation numbers.
C) A redox reaction in which atoms of an element from two oxidation states are reduced and oxidized to one single oxidation state.
D) A redox reaction that involves reduction of atoms of an element to two different oxidation numbers.
Answer: A
6) Which of the following elements DOES NOT show disproportionation reaction?
A) Mn
B) Cu
C) Cl
D) K
Answer: D
7) In living systems, superoxide dismutase, an enzyme catalyzes the disproportionation of superoxide, O2- to form:
A) H2O & O2-
B) H2O2 & O2-
C) H2O & O2
D) H2O2 & O2
Answer: D
Explanation: In the superoxide free radical ion, the oxidation state of oxygen is -1/2. It disproportionates to hydrogen peroxide (O.S. = -1) and dioxygen (O.S. = 0).
2 O2− + 2H+ → H2O2 + O2
8) Single walled carbon nanotubes are produced in high pressure carbon monoxide (HiPco) process that involve the Boudouard reaction in which carbon monoxide disproportionates to carbon and carbon dioxide on iron nano particles. The change in oxidation state of carbon is from +2 to:
A) 0 & -4
B) 0 & -2
C) 0 & +4
D) 0 & +2
Answer: D
Explanation: The disproportionation reaction of carbon monoxide is: 2CO → C + CO2
9) Consider the change in oxidation state of Bromine corresponding to different emf values as shown in the diagram below
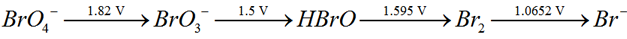
Then the species undergoing disproportionation is: (NEET 2018)
1) Br2
2) BrO4-
3) BrO3-
4) HBrO
Answer: 4
Explanation: The species that undergoes disproportionation has a more positive emf value to its right than the emf to its left in the Latimer diagram. In this case, HBrO has a more positive emf value to its right (for reduction of HBrO to Br2) than the emf to its left (for oxidation of HBrO to BrO3-). Hence it can undergo disproportionation readily.