* The disproportionation reaction of aldehydes without α-hydrogens in presence of a strong base to furnish an alcohol and a carboxylic acid is called Cannizzaro reaction. One molecule of aldehyde is reduced to the corresponding alcohol, while a second one is oxidized to the carboxylic acid.
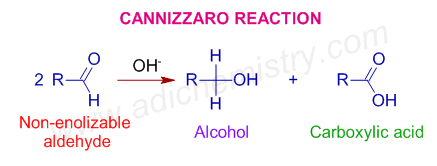
* The applicability of Cannizzaro reaction in organic synthesis is limited as the yield is not more than 50% for either acid or alcohol formed.
* In case of aldehydes that do have α-hydrogens, the aldol condensation reaction takes place preferentially.
* The α,α,α-Trihalo aldehydes undergo haloform reaction in strongly alkaline medium. E.g. Choral will give chloroform in presence of an alkali.
- Mechanism
- Illustrations & Examples
- Cross Cannizzaro reaction - Mechanism
- Intramolecular Cannizzaro reaction
- Cannizzaro reaction Practice Multiple choice questions
MECHANISM OF CANNIZZARO REACTION
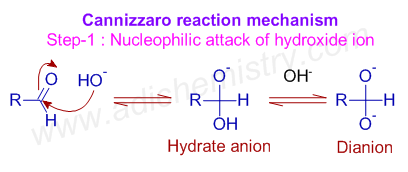
* The Cannizzaro reaction is initiated by the nucleophilic attack of a hydroxide ion on the carbonyl carbon of an aldehyde molecule by giving a hydrate anion, which can be further deprotonated to give a dianion in a strongly alkaline medium. Note that, in the second step, the hydroxide behaves as a base.
* Now a hydride ion, H- is transferred either from the monoanionic species or dianionic species onto the carbonyl carbon of another aldehyde molecule. The strong electron donating effect of O- groups facilitates the hydride transfer and drives the reaction further. This is the rate determining step of the reaction.
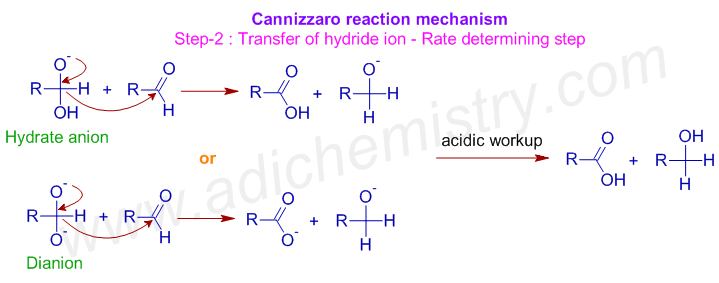
* Thus one molecule is oxidized to carboxylic acid and the other one is reduced to an alcohol.
* When the reaction is carried out with D2O as solvent, the resulting alcohol does not show carbon bonded to deuterium. It indicates that hydrogen is transferred from the second aldehyde molecule, and not from the solvent.
* The overall order of the reaction is usually 3 or 4.
* The Cannizzaro reaction takes place very slowly when electron-donating groups are present. But the reaction occurs at faster rates when electron withdrawing groups are present.
ILLUSTRATIONS & EXAMPLES OF CANNIZZARO REACTION
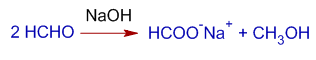
1) Formaldehyde is disproportionated to formic acid and methyl alcohol in strong alkali.
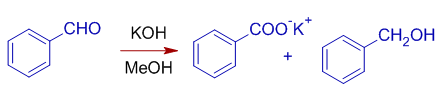
2) Benzaldehyde can be converted to benzoic acid and benzyl alcohol.
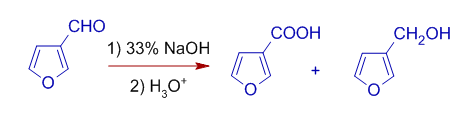
3) Furfural gives furoic acid and furfuryl alcohol in presence of strong alkali.
4) Crossed Cannizzaro reaction: When a mixture of formaldehyde and a non enolizable aldehyde is treated with a strong base, the latter is preferentially reduced to alcohol while formaldehyde is oxidized to formic acid. This variant is known as cross Cannizzaro reaction.
E.g. Benzyl alcohol and formic acid are obtained when a mixture of benzaldehyde and formaldehyde is treated with alkali.
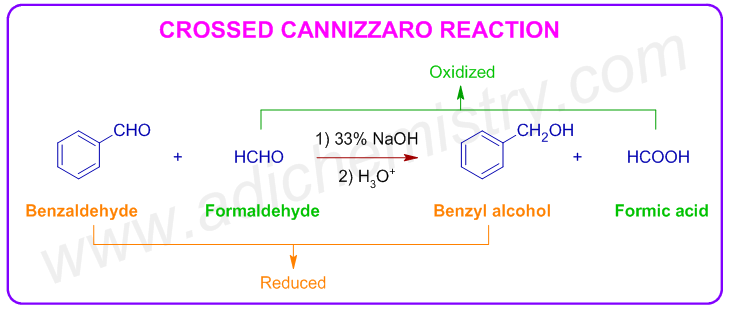
The reason may be: the initial nucleophilic addition of hydroxide anion is faster on formaldehyde as there are no electron donating groups on it.
The preferential oxidation of formaldehyde in crossed Cannizzaro reactions may be utilized in the quantitative reduction of some aldehydes.
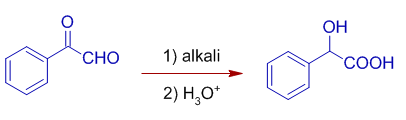
5) α-keto aldehydes can be converted to α-hydroxy carboxylic acids by an intermolecular Cannizzaro reaction.
E.g. Phenylglyoxal undergoes intermolecular cannizzaro reaction by giving Mandelic acid (α-hydroxyphenylacetic acid or 2-Hydroxy-2-phenylethanoic acid)
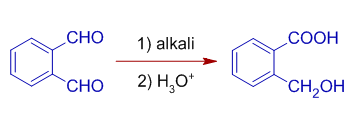
6) Phthalaldehyde can undergo intramolecular Cannizzaro reaction by giving (o-hydroxymethyl) benzoic acid.
Why does Chloral not undergo Cannizzaro reaction?
Chloral, CCl3CHO undergoes haloform reaction instead of Cannizzaro reaction to give chloroform and formate anion since the CCl3- group is a good leaving group rather than the hydride ion, H-.
CANNIZZARO REACTION PRACTICE QUESTIONS - SOLVED - IIT JEE NEET IIT JAM CSIR NET GATE
Question-1: In the Cannizzaro reaction, consider the following statements (I), (II) and (III): (CHATTISGARH SET 2017)
(I): 2 Molecules of HCHO reacts with NaOH to form CH3OH and HCOONa.
(II): The transfer of hydride ion to the carbonyl group.
(III): The attack of OH- is at the carbonyl group.
The correct from the following is:
A) (I) and (II) are correct and (II) is not an explanation of (I)
B) (I) is correct and (II) is incorrect
C) (I) and (II) are correct and (II) is an explanation of (I)
D) (II) and (III) are incorrect
Answer: C
Question-2: Which among the following will undergo Cannizzaro reaction? (KERALA SET 2012)
A) Acetaldehyde
B) Phenylacetaldehyde
C) Formaldehyde
D) Acetone
Answer: C
Question-3: Formaldehyde undergoes Cannizzaro reaction in presence of concentrated NaOH solution to form : (Rajasthan Public Service Commission 2016)
1) Methanol only
2) methanoic acid only
3) Ethanol and sodium formate
4) methanol and sodium formate
Answer: 4
Question-4: Cannizzaro reaction is not given by (IISC PHD 2010) (BHU MSC 2012)
(a) formaldehyde
(b) acetaldehyde
(c) benzaldehyde
(d) trimethylacetaldehyde (pivalaldehyde)
Answer: b
Question-5: The incorrect statement regarding Cannizzaro reaction is:
1) The rate determining step involves intermolecular transfer of hydride ion.
2) In presence of concentrated bases, the order with respect to aldehyde is 2 and w.r.t base is 2.
3) 100% yield of oxidation of benzaldehyde to benzoic acid can be achieved.
4) When formaldehyde is treated with Na18OH, the labelled oxygen is found in the product - methanol.
Answer: 3
Question-6: Cross cannizzaro reaction between formaldehyde and benzaldehyde results in a mixture of :
1) Benzyl alcohol and benzoic acid
2) Formic acid and methyl alcohol
3) Benzyl alcohol and formic acid
4) Benzyl alcohol and methanol.
Answer: 3
Explanation: Benzaldehyde reacts with formaldehyde in the presence of alkali to form benzyl alcohol and formic acid.
Question-7: In cannizaro’s reaction, the rate determining step is :
1) Intermolecular transfer of a proton
2) Intramolecular transfer of hydride ion
3) Intermolecular transfer of hydride ion
4) Nucleophilic attack of hydroxide ion on carbonyl carbon of aldehyde.
Answer: 3
Question-8: The Cannizzaro reaction is an example of :
1) Disproportionation
2) Comproportionation
3) Nucleophilic addition
4) Condensation
Answer: 1
Question-9: Crossed Cannizzaro reaction is possible between:
A) two molecules of Glyoxal
B) phenylacetone and ethanal
C) formaldehyde and benzaldehyde
D) two molecules of formaldehyde
Answer: C
Question-10: What are the products of the Cannizzaro reaction?
A) A carboxylic acid and an alcohol
B) Carboxylyic acid only
C) Alcohol only
D) An aldehyde and carboxylic acid
Answer: A
Question-11: Cross cannizzaro reaction is an example of a :
A) disproportionation reaction
B) comproportionation reaction
C) redox reaction .
D) Elimination
Answer: C