The Aldol addition reaction involves the addition of α-carbon of an enolizable aldehyde or ketone to the carbonyl group of another aldehyde or ketone and thus by giving a β-hydroxy carbonyl compound also known as an aldol (indicating both aldehyde and alcohol groups). The reaction is catalyzed more commonly by a base or some times by an acid.
If the β-hydroxy carbonyl compound containing an α-hydrogen undergoes subsequent dehydration to yield an α,β-unsaturated carbonyl compound, the entire process is also called as Aldol condensation. The dehydration step is possible under the aldol reaction conditions or mostly carried out by heating in presence of an acid or sometimes during acidic workup.
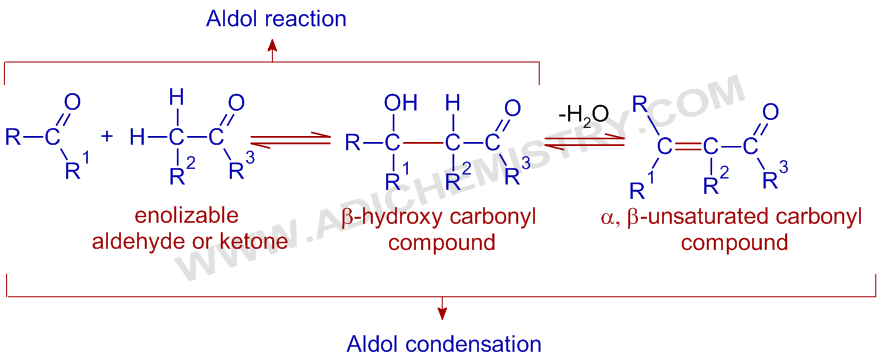
This reaction is a powerful means of making carbon-carbon bonds. The new C-C bond formed is shown in red color in above reaction sequence.
- Mechanism of aldol reaction
- Reaction conditions and control of product mixture
- Crossed aldol reactions
- Directed aldol reactions
- Examples
MECHANISM OF ALDOL REACTION
In basic medium:
* The first step in base catalyzed aldol reaction is the abstraction of an α-hydrogen from the enolizable carbonyl compound to give a resonance stabilized enolate anion. Usually this step is slow and rate determining.
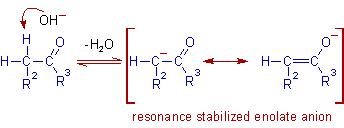
Note: Usually hydroxide ion is used as a base, since it is less basic than the enolate ion. This ensures the formation of only small amount of enolate ion and is a necessary condition for further reaction of this ion with unionized carbonyl compound. Otherwise, if strong bases like amide are used, all the reactant molecules are converted to enolate ions and no further addition is possible.
*The next step is the nucleophilic addition of enolate anion to the carbonyl group of second aldehyde or ketone molecule. The final product formed after protic workup is a β-hydroxy carbonyl compound (an aldol).
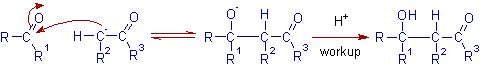
Note: In aldol reaction, the enolizable aldehyde or ketone acts as nucleophile, whereas the carbonyl group of other molecule acts as electrophilic centre.
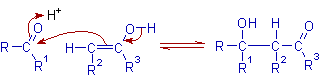
In acidic medium:
* Initially an enol is generated from the enolizable carbonyl compound during the acid catalyzed aldol reaction.
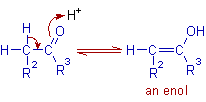
* Thus formed enol reacts with the protonated carbonyl group of another molecule.
Dehydration:
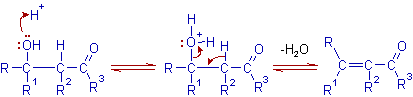
The spontaneous dehydration of β-hydroxy carbonyl compound containing an α-hydrogen may be possible under the aldol reaction conditions or by heating in acid medium. The formation of stable α,β-unsaturated carbonyl compound is the driving force for this step. Hence the initial aldol addition product is often not isolated.
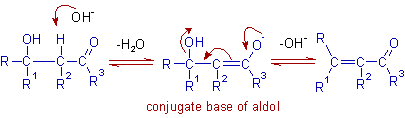
Dehydration in basic medium:
It proceeds via E1cB (Elimination Unimolecular conjugate Base) mechanism. Initially, the hydroxide ion removes acidic α-hydrogen to yield an enolate ion, which is a conjugate base of aldol. In the second step, the hydroxide ion is lost from the enolate ion to furnish the α,β-unsaturated carbonyl compound.
Dehydration in acidic medium:
ALDOL REACTION CONDITIONS & CONTROL OF THE PRODUCT MIXTURE
Aldol reaction with two same aldehyde molecules
Both the aldol reaction and condensation are reversible. In aldol reactions between two molecules of the same aldehyde are generally quite successful, since the equilibrium lies far to the right, and the yields are very high.
E.g. The formation of aldol (β-hydroxy butyraldehyde) from two molecules of acetaldehyde and subsequent dehydration to crotonaldehyde occurs readily in presence of a base like NaOH or Na2CO3; or an acid like HCl.
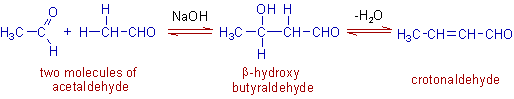
Note: There are two geometric isomers (i.e., E and Z) possible for crotonaldehyde.
Aldol reaction with two same ketone molecules
However the equilibrium lies to the left in case of ketones. The equilibrium is to be shifted to the right to achieve satisfactory yields by adjusting the reaction conditions. A Soxhlet extractor is generally employed to serve this purpose.
A mixture of products is formed in case of unsymmetrical ketones if both the groups have α-hydrogens. However such ketones react preferentially at less hindered side.
E.g. The major addition product formed when 2-butanone is treated with a base is shown below.
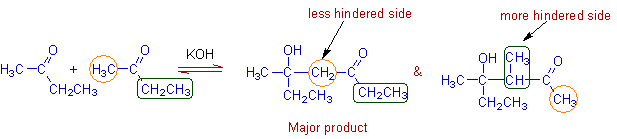
Note: However, each of above addition products undergoes dehydration easily by giving E & Z isomers of corresponding α,β-unsaturated carbonyl compound. Thus four isomeric α,β-unsaturated carbonyl compounds are formed in the reaction upon completion of reaction.
Crossed aldol reactions (Claisen–Schmidt reactions)
With two different enolizable carbonyl compounds
A mixture of addition products are formed when two different enolizable carbonyl compounds are subjected to aldol reaction conditions.
For example, four different addition products are formed (without considering the stereoisomers), when two different enolizable aldehydes are reacted i.e., two aldols from reaction between molecules of the same aldehyde and two crossed aldol products from different aldehyde molecules.
E.g. When a mixture of acetaldehyde and propionaldehyde are treated with a base, a mixture of four addition products are formed as shown below.
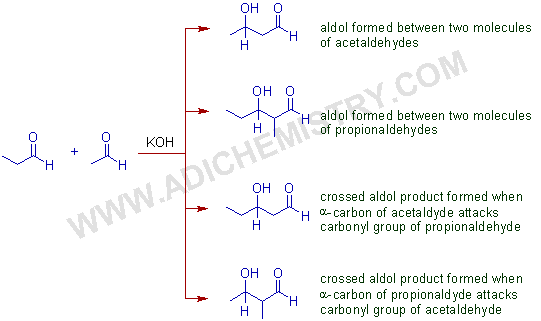
Again, each of these addition products gives two geometrical isomers upon dehydration, leading to eight different condensed products. That is why the aldol reaction between two different enolizable carbonyl compounds is seldom employed in organic synthesis.
However, the crossed aldol reactions have synthetic utility in two cases:
i) Crossed Aldol reactions with two different carbonyl compounds; one is enolizable and another is non-enolizable
Only one crossed aldol product is formed when one of the carbonyl compound lacks α-hydrogen.
E.g. Cinnamaldehyde is formed as the only crossed aldol product when acetaldehyde (enolizable) reacts with benzaldehyde (non-enolizable) in basic medium.
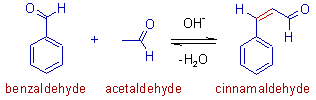
Note: In above reaction, the self aldol condensation of acetaldehyde is a competing reaction. To suppress this, along with the possible cannizzaro reaction, slow addition of acetaldehyde to benzaldehyde maintained in a mild alkaline medium is suggested.
ii) Crossed Aldol reactions between an aldehyde and a ketone.
Since the carbonyl carbon of aldehyde is more electrophilic than that of ketone, the reaction between an aldehyde an a ketone is synthetically viable. The α-carbon of ketone usually adds to the carbonyl carbon of aldehyde, leading to only one major product.
E.g. The aldol reaction of acetaldehyde with acetophenone leads to the formation of 1-phenylbut-2-en-1-one predominantly.
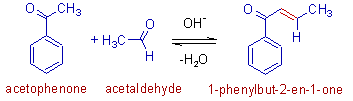
DIRECTED ALDOL REACTIONS
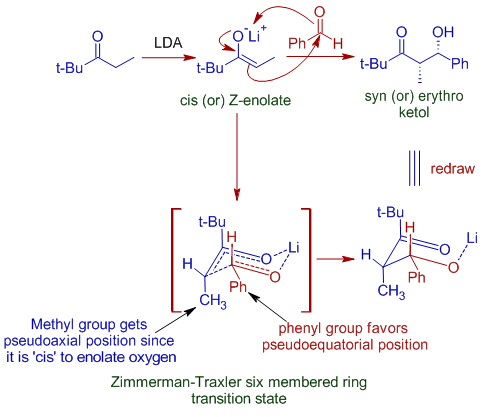
The directed aldol reactions involve preformed enolate of the carbonyl compound using strong base like LDA. The lithium enolate is treated with another carbonyl compound to achieve desired crossed aldol addition.
The lithium enolate can be either cis (Z) or trans (E). Cis enolate usually gives syn addition product and trans enolate gives anti addition product.
In most of the cases, the cis form is kinetically favored and hence syn ketol is formed selectively as the major product of addition.
This diastereoselectivity is due to the formation of cyclic six membered transition state (Zimmerman-Traxler transition state) in which the bulky group of carbonyl compound favorably takes pseudo-equatorial position, whereas the bulky group on the enolate has to take pseudo-axial position. This leads to syn product.
E.g.
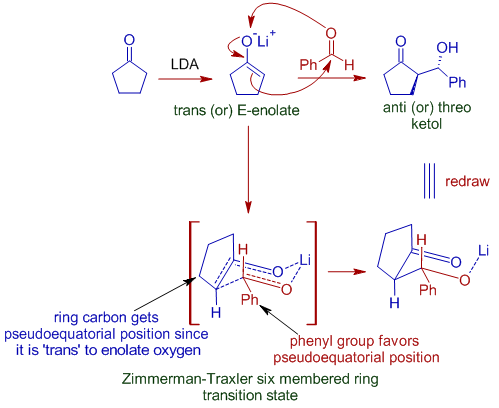
However with cyclic systems, the trans enolates are formed due to ring constraints. Hence anti addition product is formed as major product. The ring carbon must be at pseudo-axial position in the cyclic transition state. Hence the anti addition product is formed as major product.
EXAMPLES OF ALDOL REACTION
1) Acetone yields diacetone alcohol when treated with Ba(OH)2 as an addition product.
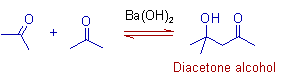
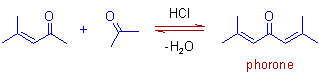
However, mesityl oxide is formed when acetone is treated with dry HCl due to subsequent dehydration of initially formed diacetone alcohol.
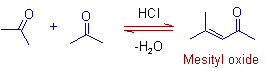
The mesityl oxide may further condense with another molecule of acetone to give phorone.
2) An intra-molecular version of aldol condensation is illustrated below with 6-oxoheptanal.
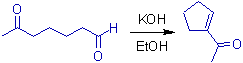
Since the carbonyl carbon of aldehyde is more electrophilic than that of ketone and five membered ring is more entropy favored than seven membered ring, the above product is formed predominantly.
ALDOL REACTION - MULTIPLE CHOICE QUESTIONS
1) Which one of the following is not likely to be formed during aldol reaction?
a) β-hydroxy carbonyl compound
b) α,β-unsaturated carbonyl compound
c) α-hydroxy carbonyl compound
d) All of the above are possible
2) Enolate formed during aldol reaction acts as a/an:
a) electrophile
b) nucleophile
c) Lewis base
d) proton donor
3) The number of aldol condensation products possible when a mixture of benzaldehyde and acetaldehyde is treated with a strong base?
a) 2
b) 1
c) 3
d) 4
4) Which of the following cannot undergo aldol reaction?
a) Methanal
b) Ethanal
c) Propanone
d) Propanal
5) In which of the following case(s), the dehydration of β-hydroxy carbonyl compound formed in the aldol reaction is NOT possible?
a) Extended conjugation in the final product i.e. α,β-unsaturated carbonyl compound.
b) Heating the reaction mixture.
c) Non-aqueous reaction conditions.
d) Absence of α-hydrogen between carbonyl and hydroxyl groups in the aldol.
6) The correct statement(s) regarding aldol reaction is/are:
a) The carbonyl group of molecule acting as electrophilic center is converted to hydroxyl group in the aldol.
b) Benzaldyhyde does not undergo aldol reaction.
c) Only one product is formed when a mixture of acetone and acetaldehyde is treated with an alkali.
d) A C-C bond is formed during elimination step.