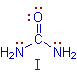
Sometimes, it is not possible to represent the molecule or ion with only one structure. More than one structure have to be proposed. But none of them explains all the observed properties of the molecule. The solution is to write a weighted average of all the valid structures, which explains all the properties. This condition is usually referred to as resonance or mesomerism or delocalization.
The representation of structure of a molecule as a weighted average of two or more hypothetical structures, which only differ by the arrangement of electrons but with same positions for atoms is referred to as resonance.
Salient features of resonance:
* The hypothetical structures with different arrangement of electrons but with identical positions for atoms are called resonance structures or canonical forms or contributing structures.
* The resonance structures are only imaginary and the actual structure of the molecule is considered as the hybrid of all the valid resonance structures.
Resonance hybrid: The weighted average of contributing structures is known as resonance hybrid. It is considered as the actual structure.
* The energy of resonance hybrid is always less than the energy of any of the contributing resonance structure.
* The resonance structures are formed (only on paper!?) due to delocalization of electrons and not by changing the positions of atoms.
* The delocalization of electrons is shown using curved arrows.
One should keep in mind that the individual resonance structures do not exist and the molecule do not resonate (switch back and forth) between these structures. The actual molecule is simply the hybrid of all these imaginary resonance structures. Hence the delocalization of electrons is also imaginary process which helps in understanding the resonance.
Illustration:
From the following structure (I) - representing urea,
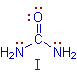
it is expected that:
1) It should be a diacidic base,
2) The bond length of C-N bond is equal to the normal C-N bond length and
3) No dipole moment since it is symmetrical.
However it is observed that:
1) Urea is a monoacidic base,
2) It has shorter than expected bond length for C-N bond and
3) It shows dipole moment.
Hence, to account for above observations, the actual structure of urea is represented as a resonance hybrid of following resonance structures.
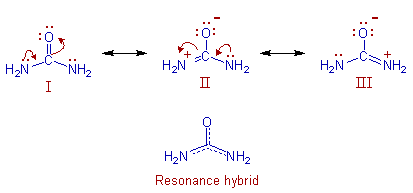
Note: The contributing structures are always shown to be linked by using
double headed arrows (![]() ).
).
The valid resonance structures must satisfy the following rules:
* They must be valid Lewis structures obeying octet rule.
E.g. Carbon or Nitrogen with five bonds is not allowed.
In the structure (II), the nitrogen atom violated the octet. It has 10 electrons around it.
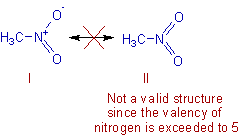
* They should possess same number of electrons and equal net charge.
* The number of unpaired electrons in them must be same.
E.g. Following structure for butadiene is not valid.
![]()
* The positions of atoms should be same in all the resonance structures.
E.g. The following are not the resonance structures, since the position of one hydrogen atom is not same. Indeed, they are different molecules, which are in dynamic equilibrium with each other. These are called tautomers. Also note that these molecules are linked by two half headed arrows and not by a single double headed arrow.
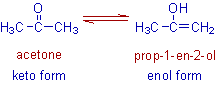
* The bond order of two connecting atoms may vary between two different resonance structures.
* The resonance structures may or may not be equivalent.
* The atoms that are part of the delocalized system must be arranged in one plane or nearly so. The reason is to get maximum overlap between the orbitals.
* The actual structure i.e., resonance hybrid of a molecule has lower energy than any of the contributing form and hence the resonance is a stabilizing phenomenon.
* Greater the number of contributing structures, greater is the stability of the resonance hybrid.
* All the structures do not contribute equally to the hybrid.
* Greater the stability of a resonance structure, larger is its contribution to the resonance hybrid.
Rules to decide the major contributor to the hybrid in the decreasing order of preference are given below:
* The contributing structures that have atoms with full octets are more stable than the ones with open octets.
* The contributing structure with more covalent bonds is more stable.
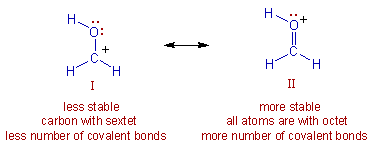
E.g. Among the following, the structure II is more stable since all the atoms have octet configuration and there are more covalent bonds.
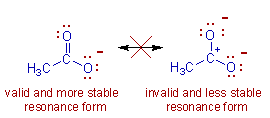
* Resonance structures with fewer charges are more stable than those with more charges.
E.g. The second structure with two negative charges is not only less stable.
* The structure with less charge separation is more stable.
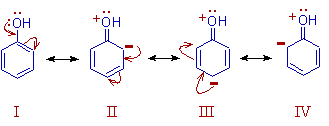
E.g. Among the following resonance forms of phenol, the structure I is more stable since it has no charge. Whereas the structures II and IV have less charge separation and are more stable than the structure III.
* The structure with charge dispersal or delocalization over more number of atoms is more stable.
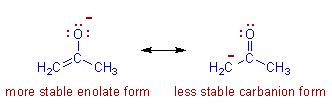
* The structures in which the atoms bearing the conventional charges are more stable i.e., the more electronegative atom should bear negative charge while the relatively less electronegative atom should bear positive charge.
E.g.
Resonance stabilization energy: The difference between the energy of resonance hybrid and that of most stable resonance structure of a molecule is known as the resonance stabilization energy of that molecule.
1) The following resonance structures can be written for benzene which are hypothetically possible due to delocalization of π electrons. The Kekule structures have more weightage than Dewar structures.
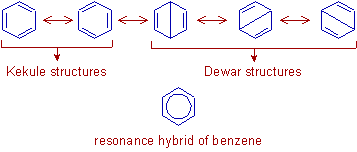
The actual structure of benzene is thus shown to be the hybrid of these contributing structures. The bond order of every C-C bond is 1.5 and hence the every C-C bond length is reported to be same and equals to 1.39 Ao, which is in between the bond length values of C-C single bond (1.54 Ao) and C=C double bond (1.20 Ao).
Due to resonance, benzene gets extra stability and does not undergo electrophilic addition reactions. However it shows electrophilic substitution reactions. This phenomenon is known as aromaticity.
Author: Aditya vardhan Vutturi