* The Clemmensen reduction is used to conveniently reduce the carbonyl compounds, which are stable to strongly acidic conditions, to alkanes.
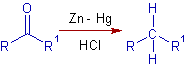
* In Clemmensen reduction, the amalgamated zinc in HCl is used as reducing agent.
* The C=O group is converted to CH2 group.
* The Clemmensen reduction is complementary to Wolff-Kishner reduction, which may be used to reduce acid sensitive compounds.
MECHANISM OF CLEMMENSEN REDUCTION
* The Clemmensen reduction occurs over the surface of zinc catalyst. The probable mechanism is shown below.
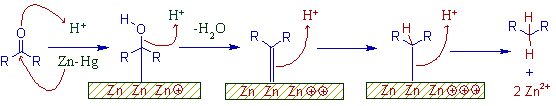
* There is a net flow of electrons from zinc to the carbonyl compound.
* As there is no formation of alcohol during the reaction, this method is not useful to reduce alcohols to alkanes.
ILLUSTRATIONS
1)
| Zn-Hg | ||||
| CH3CHO + 4(H) | -----------------> | CH3CH3 + H2O | ||
| HCl |
2) 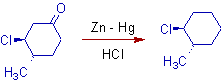
3) In the following reaction, along with the reduction of carbonyl group, the -OH group is substituted by the -Cl group (side reaction). However this side reaction can be avoided by employing Wolff-Kishner method.
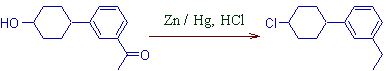
4) But the phenol group is not affected in Clemmensen reduction. (Why? Ans: Nucleophilic substitution is not easy on sp2 carbon of benzene ring!)
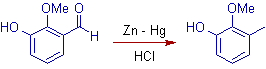
GRIGNARD REACTION - MCQ - IIT JEE - NEET - JAM - GATE - CSIR NET & SET EXAMS
1) Acetophenone when reacted with Zn (Hg) and HCl in ethyl alcohol easily gives ethyl benzene. This is an example of : (KERALA SET 2010)
A) Clemmensen reduction
B) Birch reduction
C) Wolf-Kishner reduction
D) MPV reaction
KEY & EXPLANATION
1) A