* In Wolff-Kishner reduction, the carbonyl compounds which are stable to strongly basic conditions can be reduced conveniently to alkanes. The C=O group is converted to CH2 group.
The carbonyl compound is first treated with excess of hydrazine to get the corresponding hydrazone which upon heating, in presence of a base, furnishes the hydrocarbon.
A high-boiling hydroxylic solvent, such as diethylene glycol (DEG), is commonly used to achieve the temperatures needed.
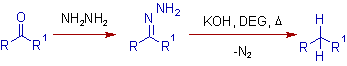
* The Wolff-Kishner reduction is complementary to Clemmensen reduction, which is used to reduce base sensitive compounds.
MECHANISM OF WOLFF KISHNER REDUCTION
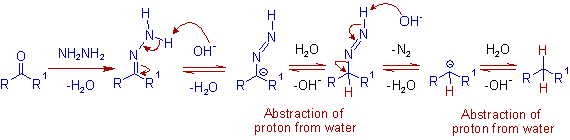
ILLUSTRATIONS
1) The Wolff-Kishner reduction of acetone gives propane.
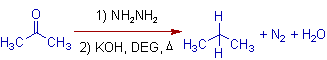
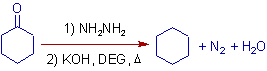
2) The cyclohexane is formed upon Wolff-Kishner reduction of cyclohexanone.
3) The Wolff-Kishner reduction is best suited for the reduction of carbonyl compounds containing groups stable to strongly basic conditions. In the following example, the alcohol group is not affected during the reduction.
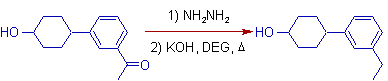
4) However, the base sensitive groups may be affected during Wolff-Kishner reduction. In the following case, the halogen group undergoes dehydrohalogenation under strongly basic conditions.
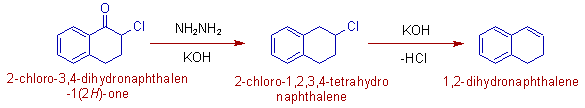
This side reaction can be avoided by using Clemmensen reduction.