* In Wurtz reaction, two alkyl halide molecules are coupled in presence of sodium metal in anhydrous ether or Tetrahydrofuran to form a new carbon carbon bond and thus by giving a symmetrical alkane.
| Dry Ether | ||
| R-X + 2Na + X-R | -----------------> | R-R + 2NaX |
Where X = halogen
* The Wurtz reaction must be performed under anhydrous conditions because the alkyl free radical formed (see the mechanism) during the reaction is strongly basic and can abstract proton from water.
* In case of alkyl and aryl fluorides as well as aryl chlorides, tetrahydrofuran is used as solvent instead of ether.
* The Wurtz reaction is limited to synthesis of symmetrical alkanes with even number of carbon atoms only. The number of carbons in the alkane is double that of alkyl halide (n ---> 2n type reaction)
* If dissimilar alky halides are used, a mixture of alkanes is formed. It is usually difficult to separate the mixture and hence wurtz reaction not a suitable method to synthesize unsymmetrical alkanes.
E.g. The Wurtz reaction between R-X and R'-X yields not only R-R' but also R-R and R'-R'. This mixture cannot be separated easily.
* Methane cannot be prepared by this method.
* A modification of this reaction involving alkyl and aryl halides is called Wurtz-Fittig reaction. If only aryl halides are subjected to coupling, the reaction is called as Fittig reaction.
MECHANISM OF WURTZ REACTION
* Initially an alkyl free radical is formed due to transfer of one electron from sodium atom.
| R-X | + | Na | ---------> | R. | + | X- |
| Alkyl free radical |
* In the next step, one more electron is transferred from second sodium atom to the free radical to give a carbonium ion.
| R. | + | Na | ---------> | R-Na+ | ||
* Thus formed alkyl anion displaces halide ion from the second molecule of alkyl halide. It is an SN2 reaction.
| R- Na+ | + | R-X | ---------> | R-R | + | Na+X- | |
| Symmetrical Alkane |
Comment: Since the alkyl free radicals are formed, elimination side reactions leading to alkenes is also possible, especially with bulky alkyl groups, which require more activation energy during the nucleophilic substitution (SN2) step.
ILLUSTRATIONS
1) Ethane is formed when methyl chloride is treated with sodium metal in dry ether.
| Dry Ether | ||
| CH3-Cl + 2Na + Cl-CH3 | -----------------> | CH3-CH3 + 2NaCl |
2) Strained carbon skeletons like bicyclobutane ( bicyclo[1.1.0]butane ) can be prepared by an intramolecular Wurtz reaction as shown below.
![preparation of bicyclo[1.1.0]butane by wurtz reaction](wurtz1-1.gif)
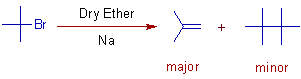
3) When tert-butylhalides are subjected to Wurtz reaction, isobutylene is formed as the major product. It is because the elimination is favored over SN2 mechanism. The SN2 step requires more activation energy due to steric hindrance.