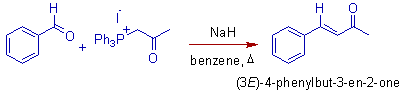The reaction of an aldehyde or ketone with a phosphonium ylide to an alkene and a phosphine oxide is known as Wittig reaction or Wittig Olefination reaction.
This reaction was discovered in 1954 by Georg Wittig, for which he was awarded the Nobel Prize in Chemistry in 1979.
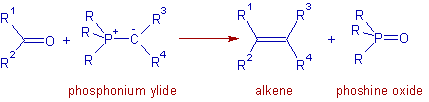
* The yields of di- and tri-substituted alkenes from aldehydes and ketones are very high but yields of tetra-substituted alkenes from ketones are often poor because of steric effects.
PHOSPHONIUM YLIDES (WITTIG REAGENTS)
* The phosphonium ylides or alkylidene phosphoranes, also known as Wittig reagents, can be prepared by treating tri-substituted phosphines with the alkyl halide to give phosphonium salts, which furnish the ylides upon treatment with the base.
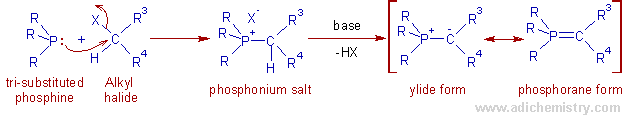
The suitable bases include NaH, NaOMe, NEt3, BuLi etc.,
The ylides are resonance stabilized structures and usually colored intensely. One of the contributing structure is a zwitter ionic form with positive and negative charges on adjacent atoms. These are prepared in solutions and are not generally isolated. Hence usually the ylide is generated in situ during the Wittig reaction.
The ylides are of two types based on their relative stability.
i) Non-stabilized ylides: The ylides with electron donating groups on negatively charged carbon are less stable and react faster. They also react with dioxygen. Hence the Wittig reaction with non-stabilized ylides is performed under inert atmosphere.
ii) Stabilized ylides: The ylides with electron withdrawing groups adjacent to the negatively charged carbon are more stable. These are usually stabilized by conjugation.
* It is generally observed that the geometry of the final alkene depends on the stability of the ylide.
* The unstabilized ylides react faster and lead to (Z)-alkenes.
* The stabilized ylides react slowly and lead to (E)-alkenes.
MECHANISM OF WITTIG REACTION
The mechanism of Wittig reaction is not fully established. However a simplified picture is given below. The initial step is the nucleophilic addition of negatively charged carbon of ylide onto the carbonyl carbon to give a betaine, which can cyclize to give an oxaphosphetane as an intermediate. The oxaphosphetane is decomposed to give an alkene and a phosphine oxide.
The driving force of the Wittig reaction is the formation of highly stable double bond between phosphorus and oxygen in phosphine oxide. The last step involves the elimination of phosphorus and oxygen through a syn-periplanar transition state. Hence this step is stereospecific.
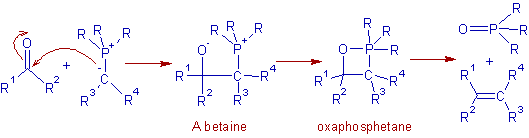
Though the formation of betaine is not established, the formation of the four membered oxaphosphetane intermediate is confirmed by 31P-NMR experiments. Hence now it is believed that the initial addition is concerted to give the oxaphosphetane directly.
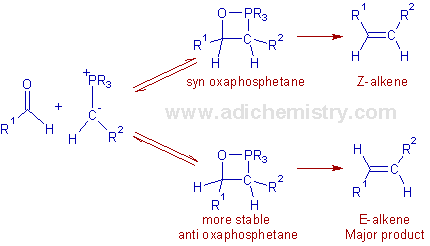
Stereochemical perspective: Both syn and anti diastereomeric oxaphosphetanes are possible. However the formation of oxaphosphetane is stereoselective depending on the conditions.
With unstabilized ylides: The Wittig reaction with unstabilized ylides yields Z-alkenes predominantly (Z-selective). This selectivity can be explained as follows:
The carbonyl compound and the ylide approach each other at right angles and form the puckered four membered oxaphosphetane ring in the transition state, in one step. The transition state is formed such that the large substituents are kept away from each other leading to the formation of a syn-oxaphosphetane which is less stable but formed very quickly than the corresponding anti diastereomer. Hence finally the kinetically controlled Z-alkene is formed.
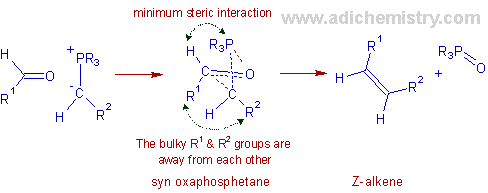
With stabilized ylides: The Wittig reaction with stabilized ylides is E-selective. Since the ylide is stable and the formation of oxaphosphetane from the starting compounds is reversible, an equilibration is possible between relatively less stable syn form and more stable anti form of oxaphosphetane. There is now much time for the syn oxaphosphetane to interconvert to more stable anti form before the decomposition occurs. Hence the E-alkene predominates. Thus the selectivity of the final product is thermodynamically controlled.
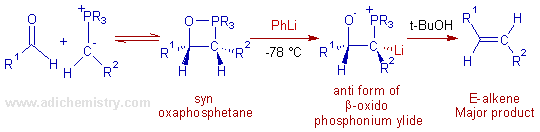
The Schlosser modification: It is possible to get E-alkenes from non-stabilized ylides using this method (Wittig-Schlosser reaction).
In Schlosser modification, the initially formed less stable syn betaine can be converted to anti form by treating with phenyllithium or n-butyllithium at very low temperatures (-78 oC). The treatment of the oxaphosphetane with these bases results in deprotonation at carbon adjacent to the phosphorus and give a more stable anti form of β-oxido phosphonium ylide. This will furnish E-alkene finally upon protonation with an acid.
Horner–Wadsworth–Emmons reaction: This is modified Wittig reaction in which carbanions generated from phosphonate esters are used instead of phosphonium ylides. This reaction is more superior to Wittig reaction since the carbanion generated from phosponates is more nucleophilic and the phosphate byproducts are water soluble and can be removed easily. It often gives better yields.
Horner–Wadsworth–Emmons reaction is also known as: Horner–Emmons or Wadsworth– Emmons or Horner–Wittig reaction.
ILLUSTRATIONS
1) In the following Wittig reaction, the cyclohexanone is converted to methylidenecyclohexane by treating with (methylene)triphenylphosphorane, which in turn is generated in situ by treating the triphenylphosphine with methylbromide in presence of a base.
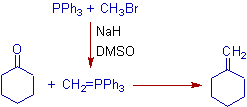
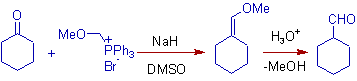
2) In the following example, the Wittig reagent is derived from the α-haloethers. They react with aldehydes or ketones to form vinyl ethers, which upon subsequent hydrolysis yield aldehydes containing one more carbon atom. Thus the conversion of cyclohexanone to cyclohexane carbaldehyde is achieved.
3) An exocyclic double bond can be successfully introduced on camphor by treating it with methyltriphenylphosphonium bromide in presence of potassium tert-butoxide.
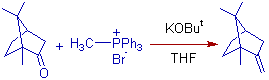
4) The Wittig reaction of propanal with butyltriphenylphosphonium iodide, a non-stabilized ylide yields (3Z)-hept-3-ene selectively.
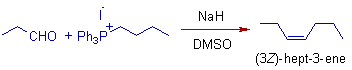
Note: Above Wittig reagent is non-stabilized since the butyl group is electron donating group.
5) But the E-selectivity is observed in the following Schlosser modification.
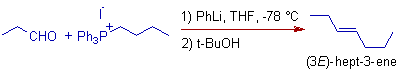
6) Whereas E-alkenes are formed predominantly with stabilized ylides as shown in the following Wittig reaction.