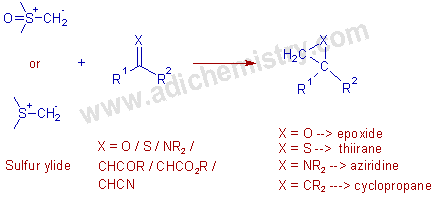
* The Corey-Chaykovsky reaction or sometimes known as Johnson-Corey-Chaykovsky reaction is employed in modern organic synthesis to prepare three membered rings like epoxides (oxiranes), thiiranes, aziridines and cyclopropanes by reacting sulfur ylides with electrophiles such as carbonyls, thiocarbonyls, imines and olefins.
Dimethylsulfoxonium methylide (Corey’s ylide) or dimethylsulfonium methylide are the common sulfur ylides used in this reaction.
SULFUR YLIDES
* The sulfur ylides are generated in situ by the deprotonation of sulfonium halides with strong bases like NaH in solvents like DMSO.
E.g. The corey ylide, dimethylsulfoxonium methylide can be generated easily by treating trimethylsulfoxonium iodide with NaH in DMSO.
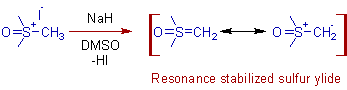
* The sulfur ylides are stabilized when electron withdrawing groups are attached to it. Usually the groups like phenyl, carbonyl, ester etc., stabilize the ylide. Whereas the electron releasing groups like alkyl tend to destabilize the ylides.
E.g. The dimethylsulfoxonium methylide is more stable than dimethylsulfonium methylide due to presence of S=O group.
Sometimes the course of the reaction is influenced by the stability of the sulfur ylides.
MECHANISM OF COREY CHAYKOVSKY REACTION
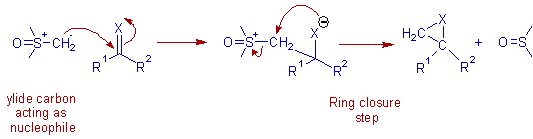
* In the first step, the ylide carbon, bearing negative charge, acts as nucleophile and makes bond with electrophilic carbon attached to 'X' atom.
* Now the ylide carbon becomes electrophilic and is attacked by the negatively charged 'X' atom, acting as intramoleuclar nucleophile to achieve ring closure to furnish a three membered ring.
* The sulfoxide (or sulfide) moiety acts as leaving group. The overall reaction is a 1,2 addition.
Reaction of sulfur ylides with Michael acceptors - kinetic vs thermodynamic control (1,2 addition vs 1,4 addition)
* When Michael acceptors are used, the choice of products formed in Corey-Chaykovsky reaction depends on the stability of sulfur ylides.
With unstabilized ylides
* Usually 1,2-addition products are formed predominantly when unstabilized ylides are used.
E.g. The kinetically favored epoxide is formed when dimethylsulfonium methylide (an unstabilized sulfur ylide) is made to react with methyl vinyl ketone, a Michael acceptor.
The epoxide is formed due to irreversible 1,2 addition directly at C=O, which occurs faster than 1,4-addtion. The intermediate formed is unstable and undergoes immediate ring closure due to intramolecular displacement of sulfide leaving group by nucleophilic oxygen leading to formation of epoxide.
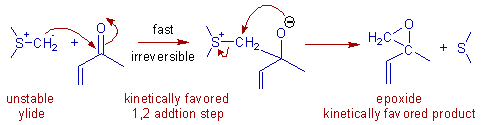
With stabilized ylides
* However, with stabilized ylides the reaction takes the 1,4-addition route and furnishes cyclopropanes (thermodynamic product) upon ring closure and is referred to as a Michael Initiated Ring Closure (MIRC) type reaction.
E.g. With dimethylsulfoxonium methylide (a stabilized sulfur ylide) cyclopropanation occurs instead of epoxidation on methyl vinyl ketone.
Cyclopropane ring is formed rather than epoxide due to 1,4-addition.
However the 1,2-addition also occurs even more faster with stabilized ylides. Since the intermediate is stable, this step is also reversible. Hence the sulfonium ylide is re-expelled before the formation of epoxide.
Meanwhile, some ylide adds to methyl vinyl ketone in 1,4 fashion irreversibly as in Michael reactions. This step is irreversible because of formation of stronger C-C σ bond at the expense of weaker π bond of C=C. This step is followed by intramolecular ring closure to give cyclopropane as a thermodynamically favored product.
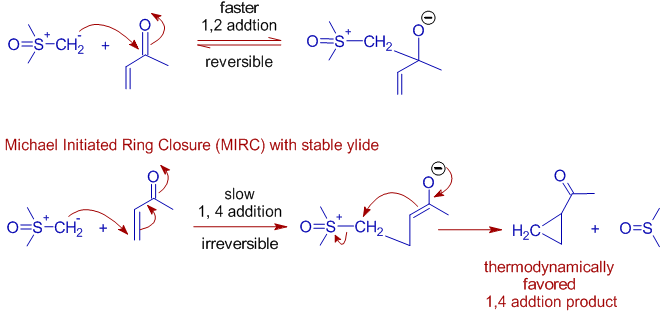
In short, with Michael acceptors, epoxidation is major route when unstable ylides are used, whereas cyclopropanation is the major route with stable ylides.
And also remember that, whether the ylide is stable or not, only epoxide is formed when the double bond is not in conjugation (unless the double bond is very much activated by EWG groups).
STEREOCHEMISTRY
The Corey-Chaykovsky cyclopropanation reactions are usually diastereoselective i.e., and both (E)- and (Z)-olefins give the trans cyclopropanes. This is because the bond rotation is faster than the ring closure as illustrated below.
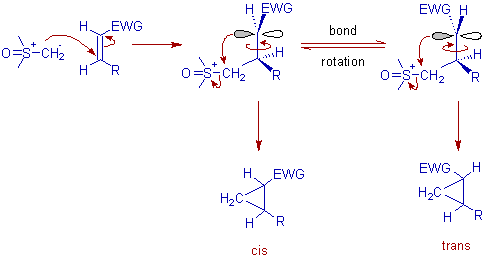
However, the stereochemistry of olefin is maintained i.e., cis-olefins give cis cyclopropanes and trans-olefins give trans cyclopropanes when the bond rotation is restricted or slow. This is observed when diphenylsulfonium methylide derivatives, Ph2S=CH2 are used as sulfur ylides.
ILLUSTRATIONS
1) Benzaldehyde can be converted to 2-phenyloxirane by treating it with dimethylsulfonium methylide, which is generated in situ from trimethylsulfonium iodide and NaH in DMSO.
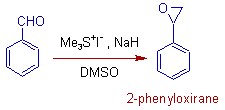
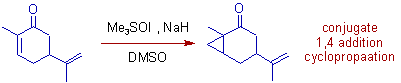
2) Epoxidation of Carvone occurs when it is treated with unstabilized ylide, dimethylsulfonium methylide. It is direct 1,2 addition on C=O.
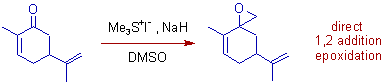
Whereas, cyclopropanation occurs when a stabilized sulfur ylide, dimethylsulfoxonium methylide is used. It is an example of conjugate 1,4 addition (MIRC).
3) In the following MIRC type of reaction on acrolein with a stable sulfur ylid, the expected trans product is formed predominantly.
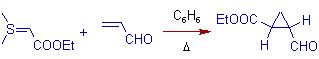
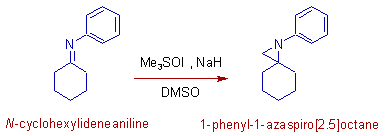
4) Imines react with sulfur ylides to give aziridines. The following reaction illustrates the formation of an aziridine, 1-phenyl-1-azaspiro[2.5]octane from an imine, N-cyclohexylideneaniline.