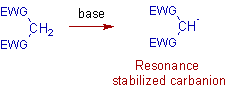
* The Michael reaction is the conjugate 1,4-addition of a resonance stabilized carbanion (michael donor) to an activated α,β-unsaturated compound (michael acceptor).
The Michael donors contain active -CH2 (methylene) group or -CH group. The acidic nature of methylene group is enhanced by the electron withdrawing groups (EWG) like: keto, cyano, nitro, carboxylic acid derivatice etc.
E.g. Active methylene compounds like Malonates (e.g.Diethyl malonate), β-keto esters (e.g.Acetoacetic ester) etc., are some of the examples for Michael donors.
Not only α,β-unsaturated ketones, however, and also esters; nitriles; sulfones; and compounds with activated double bonds can act as Michael acceptors. Vinyl ketones, alkyl acrylates, acrylo nitrile, fumarates etc., are some examples.
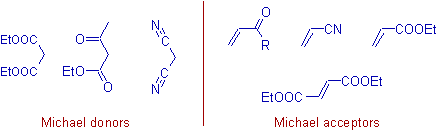
* A strong base is employed to generate enolate ion from Michael donor. The bases used in the reaction include: alkoxides, LDA, NaOH, KOH etc., in protic solvents like alcohols.
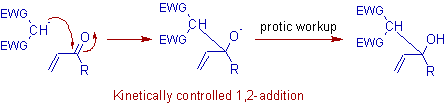
* Michael addition is a thermodynamically controlled conjugate 1,4 addition reaction and competes with kinetically controlled 1,2 addition to C=O. At low temperatures, 1,2 additon occurs predominantly. But at higher temperatures, the michael addition is the preferred route.
MICHAEL ADDITION : MECHANISM
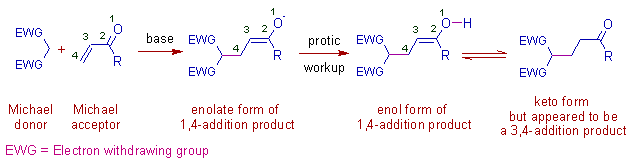
* Initially a resonance stabilized enolate ion (nucleophile) is produced from Michael donor in presence of a strong base.
It is added to the 4th carbon of α,β-unsaturated carbonyl compound and thus by giving a new enolate, which usually yields the final compound upon hydrolytic workup.
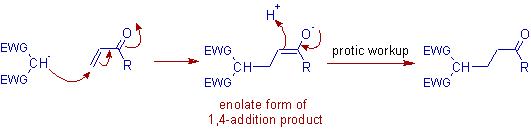
Note: The final product formed in protic workup appears to be a 3,4-addition product.
* The conjugate 1,4-addition product is thermodynamically controlled and occurs predominantly at relatively higher temperatures. But a kinetically controlled 1,2-addition is also possible which is preferred at low temperatures as shown below.
ILLUSTRATIONS
1) Michael addition of diethyl malonate with methyl vinyl ketone followed by protic workup yields a 1,5-dicarbonyl compound.
Note: Hydrolysis of ester groups and decarboxylation occurs in the final step.
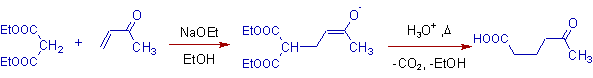
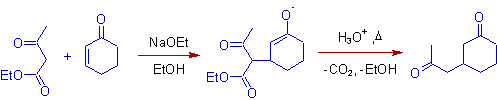
2) Ethyl acetoacetate (acetoacetic ester) can be used as michael donor.
3) In the following reaction, Michael addition of diethyl malonate to mesityl oxide yields a 5-Oxocarboxylic acid.
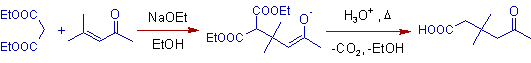
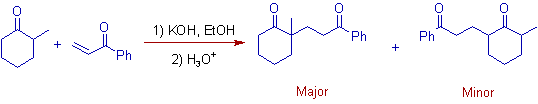
4) Usually more substituted alpha-carbon of Michael donor is involved in the addition.
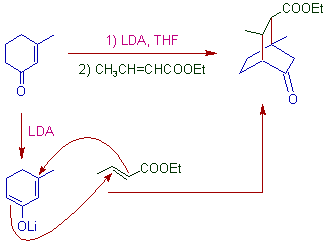
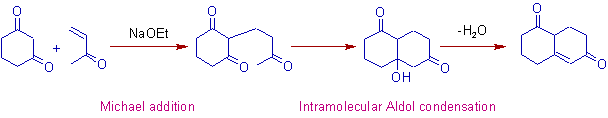
5) Robinson annulation involves, the Michael addition followed by intramolecular aldol condensation as illustrated below.
6) Both cis and trans isomers are possible in Michael addition involving alkynes.

7) Michael additions to extended conjugate systems is also observed. Following is an example of 1,6-addition.
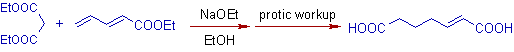
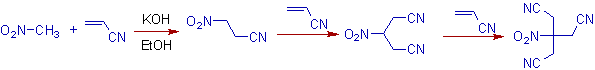
8) Nitroalkanes with α-hydrogens are excellent Michael donors. The Michael addition of nitromethane with acrylonitrile is one such example. In this case, three moles of acrylonitrile are involved in the addition.
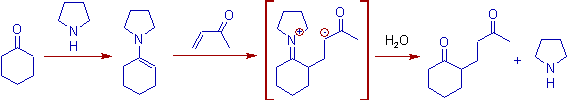
9) Enamines are excellent Michael donors. These are usually generated by treating carbonyl compounds with pyrrolidine. The reaction of enamines with michael acceptors is also known as Stork-Enamine reaction.
10) Bicyclo[2.2.2]octane systems can be prepared by double michael addition as illustrated below.