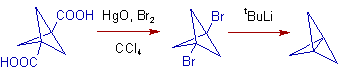The decarboxylation of silver salts of carboxylic acids to alkyl bromides by treating with bromine is known as Hunsdiecker reaction. The alkyl bromide contains one carbon less than those in carboxylic acid.
This reaction is also known as Borodin-Hunsdiecker reaction.
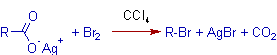
* Very good yields are obtained with alkyl groups containing 2 to 18 carbons. This reaction works with linear as well as branched chains. However the reaction seldom works with alkyl groups containing unsaturation.
* This reaction is usually carried out in carbon tetrachloride solvent.
* Although bromine is used often, the reaction is also possible with chlorine and iodine.
* When iodine is used, the ratio between the silver carboxylate and iodine is very important and determines the products.
A 1:1 ratio of silver salt and iodine gives the alkyl halide.
However, an ester, RCOOR is formed when the reaction is carried out with a 2:1 ratio of silver carboxylate and iodine. This is called as Simonini reaction.
* Incase of aromatic carboxylates, the Hunsdiecker reaction is possible when the aromatic ring contains electron-withdrawing groups.
Otherwise, if the aromatic system contains electron-donating groups, the bromine will substitute one of the hydrogen on the aromatic ring rather than promoting the Hunsdiecker reaction.
However the use of NBS instead of bromine will give the desired Hunsdiecker product. This reagent is especially useful since it produces bromine free radicals slowly.
* The silver carboxylate used as the starting material must be sufficiently pure and dry. It can be prepared from the corresponding carboxylic acid by treating it with silver oxide, Ag2O.
Christol-Firth Modification: It is possible to perform the Hunsdiecker reaction conveniently on the free carboxylic acid instead of the silver salt, which otherwise requires purification. In this modification the free carboxylic acid is treated with a mixture of mercuric oxide, HgO and bromine in CCl4. There is no need to isolate an intermediate salt.
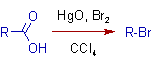
MECHANISM OF HUNSDIECKER REACTION
Initiation: Initially the bromine reacts with the silver carboxylate to give an unstable acyl hypobromite. The driving force of this step is the precipitation of the extremely poorly soluble and stable AgBr.
The acyl hypobromite decomposes by homolytic cleavage of relatively weak O-Br bond to furnish an acyl free radical.
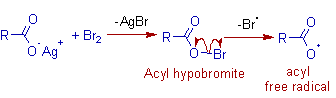
Propagation: The acyl free radical undergoes decarboxylation to furnish an alkyl free radical, which reacts with acyl hypobromite to give the final product alkyl bromide along with the formation of a new acyl free radical.
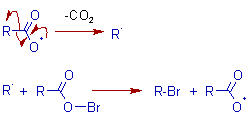
The following facts support the above proposed free radical mechanism for Hunsdiecker reaction.
i) No rearrangement of alkyl groups
ii) The formation of side products like R-R.
iii) If the alkyl group, R is chiral, it loses its optical activity during this reaction.
ILLUSTRATIONS
1) The silver salt of propionic acid is converted to ethyl bromide when treated with bromine in tetrachloromethane.
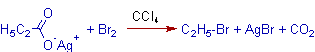
2) In the following reaction, the use of NBS (N-Bromosuccinimide) reduces the chances of electrophilic substitution on benzene ring.
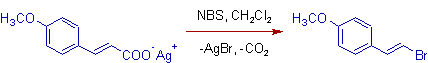
3) The Christol-Firth Modification is used in the preparation of [1.1.1]propellane (tricyclo[1.1.1.01,3]pentane). The conversion of Bicyclo[1.1.1]pentane-1,3-dicarboxylic acid to the corresponding dibromide is achieved by using mercuric oxide and bromine in carbon tetrachloride as shown below.