The Friedel-Crafts alkylation involves the electrophilic substitution of alkyl groups on aromatic rings when arenes are treated with alkyl halides in presence of Lewis acids.
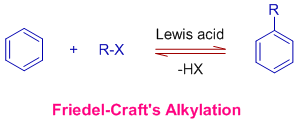
* The alkenes or alcohols can also be used to alkylate aromatic rings under Friedel-Crafts conditions.
* This reaction is catalyzed by Lewis acids like anhydrous AlCl3, FeX3, ZnCl2, BF3 etc.
* It is a reversible reaction and hence dealkylation is also possible under the above said conditions.
* The Friedel-Crafts alkylation fails when the aromatic systems contain more powerful electron withdrawing groups than halogens (like nitro group).
Aniline does not undergo alkylation since the lone pair on nitrogen of amino group forms coordinate bond with AlCl3, preventing the complexation to alkyl halides.
* The aryl and vinyl halides cannot be used instead of alkyl halides.
MECHANISM OF FRIEDEL CRAFTS ALKYLATION
1) Initially, a complex is formed due to coordination of alkyl halide to the Lewis acid. This complex may act as the electrophilic reagent or it may dissociate to give a free carbocation, which can act as electrophile.
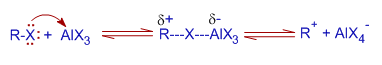
2) The next step involves the addition of the complex or the carbocation to the aromatic system to give a σ-complex, which loses a proton subsequently to reconstitute the aromatic system.
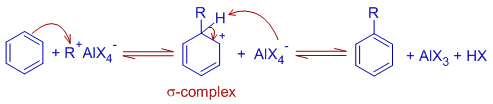
* The π electrons on aromatic ring act as nucleophilic system. They attack the electron deficient carbocation to form the σ-complex.
* The σ-complex formed is also resonance stabilized. However it is not as stable as the initial aromatic system.
* The carbocation may rearrange to give unexpected products.
* The carbocations may also be generated either i) by protonating the alkenes or ii) from alcohols by dehydration.
* Since the product formed is highly activated towards the electrophilic substitution, further alkylation is also possible leading to formation of poly alkylated products. This is one of the drawback of this reaction.
ILLUSTRATIONS
1) Toluene is formed when benzene is treated with methyl chloride (chloromethane) in presence of anhydrous aluminium chloride.
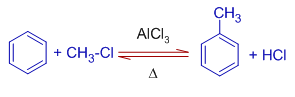
However, toluene is even more reactive than benzene and hence may undergo further substitution to give a mixture of polymethylated benzenes.
2) Cumene (Isopropylbenzene) is formed as major product when benzene is treated with propyl bromide at 80oC in presence of anhydrous aluminium chloride.
The propyl carbocation is rearranged to more stable isopropyl carbocation by hydrogen ion shift during the reaction. Hence the major product is cumene rather than propyl benzene.
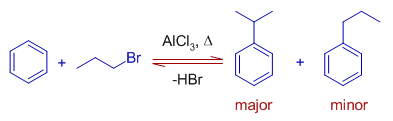
Note: However if the reaction is carried out at room temperature, the major product is propyl benzene.
3) The alkenes can also be used to generate the electrophilic carbocation. The carbocation is generated by using a protic acid. The proton is added to the the doubly bonded carbon so as to generate the more stable carbocation.
E.g. Cumene can also be prepared by the when benzene is treated with propylene gas under 30 atm of pressure in presence of H3PO4. Again the stable secondary carbocation is generated from the alkene during the reaction. Sulfuric acid can also be used as catalyst.
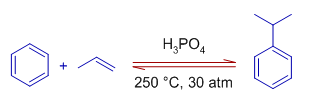
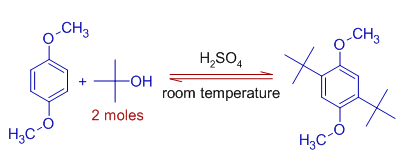
4) The alcohols are also used to generate the carbocations which may further participate in Friedel-Crafts reaction.
E.g. 1,4-dimethoxybenzene reacts with t-butyl alcohol to form 1, 4-Di-t-butyl-2, 5–dimethoxybenzene in presence of sulfuric acid acting as catalyst. In this reaction, t-butyl carbocation is generated via dehydration of protonated alcohol.
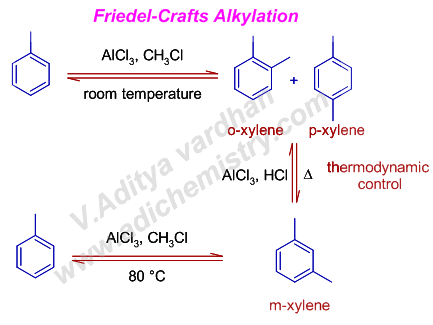
5) The Friedel-Crafts alkylation is a reversible reaction unlike acylation. Hence a sequence of alkylation and dealkylation steps are possible through out the reaction. This may sometimes lead to unexpected products under thermodynamic control conditions such as prolonged reaction times or at high temperatures.
E.g. Toluene undergoes ortho and para substitutions when treated with chloromethane at room temperature to give ortho and para xylenes. However meta xylene is formed as the major product when the reaction mixture is heated to 80 oC.
In fact, meta xylene is obtained when either ortho xylene or para xylene is heated with AlCl3/HCl mixture due to rearrangement which involve dealkylation and alkylation steps.
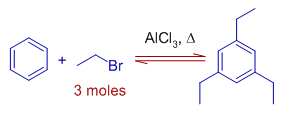
6) When benzene is made to react with ethyl bromide at room temperature for several hours, the final major product formed is thermodynamically more stable 1,3,5-triethylbenzene. The meta substitutions occur due to less steric interactions.
The initially formed mono substituted product directs the orientation for second ethyl group at ortho and para positions. However these kinetically favored but thermodynamically less stable products may undergo dealkylation and prefer to take the meta position during the prolonged hours of the reaction.
7) Durene (1,2,4,5-tetramethyl benzene) is obtained as major product when para xylene is reacted with chloromethane under Friedel-Crafts conditions.
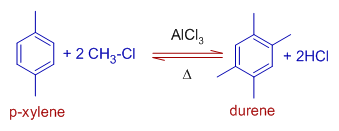
The pentamethyl and hexamethyl benzenes are also formed as side products.
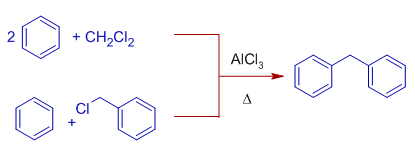
8) Diphenylmethane is obtained when an excess of benzene is reacted with dichloromethane in presence of AlCl3. The two chlorine atoms of dichloromehtane (methylene chloride) are replaced by benzene rings.
The same product is obtained when benzene is treated with benzene under Friedel-Crafts conditions.
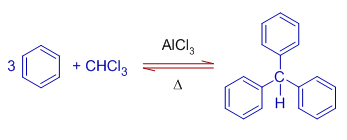
9) Triphenylmethane can be prepared by reacting excess of benzene with trichloromethane (chloroform) in presence of anhydrous aluminium chloride.
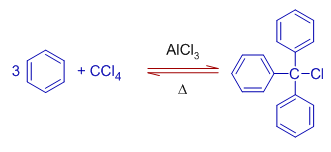
10) However Triphenyl chloromethane (Tritylchloride) is obtained when excess of benzene reacts with tetrachloromethane (carbon tetrachloride) under Friedel-Crafts conditions. The replacement of fourth halogen atom is not possible due to steric hindrance.
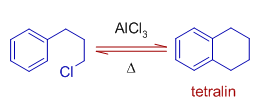
11) The formation of tetralin in following reaction involves intramolecular Friedel-Crafts reaction.
FRIEDEL CRAFTS ALKYLATION - PRACTICE QUESTIONS - MCQ - IIT JEE NEET IIT JAM CSIR NET GATE CHEMISTRY
1) What is the electrophile in the Friedel-Crafts alkylation reaction with Isopropylchloride?
A) the isopropyl cation
B) a complex of isopropylchloride and aluminum chloride
C) a proton
D) aluminum chloride
Hint: Electrophile is a lewis acid.
2) How can polyalkylation be minimized in Friedel-Crafts alkylation?
A) Use an alkyl halide without a Lewis acid catalyst.
B) Use a large excess of aromatic compound relative to the alkyl halide.
C) Use a large excess of alkyl halide relative to the aromatic compound.
D) Use a large excess of the Lewis acid catalyst.
Hint: Electrophile is a lewis acid.
3) Which of the following halides cannot be used as the electrophile for a Friedel-crafts alkylation reaction?
A) Chloromethane
B) 2-Bromopropane
C) Iodobenzene
D) Ethylbromide
Answer: C
4) The reaction in which benzene reacts with alkyl halide in the presence of a lewis acid as a catalyst to produce alkylbenzene is known as ___________
a) Nitration
b) Friedel-Crafts Alkylation
c) Friedel-Crafts Acylation
d) Halogenation
e) Sulfonation
Answer: b
5) Which of the following aromatic compounds undergo Friedel–Crafts alkylation with ethyl bromide in presence of aluminum bromide?
a) Benzoic acid
b) Nitrobenzene
c) Toluene
d) Aniline
Answer: c
6) Which of the following is an incorrect statement?
a) Friedel-Crafts alkylation can be reversible.
b) Reaction of propylchloride with benzene in presence of AlCl3 involves rearrangement of intermediate carbocation.
c) Methylation of benzene with an methyl chloride requires only a catalytic amount of a Lewis acid.
d) Only monosubstitution is possible when toluene reacts with excess of alkyl chloride in presence of AlCl3.
Answer: d
7) Reaction of 1,3-dimethylbenzene with tert-butyl chloride in presence of AlCl3 occurs primarily at carbon 5 because:
a) Methyl groups are ring deactivating towards electrophilic aromatic substitution.
b) In general, the methyl groups are meta directing groups..
c) The normally more rapid substitution at the other positions is slowed by steric hindrance
d) The alkyl groups are ortho and para directors.
Answer: c
8) The reaction of benzene with methyl chloride under Friedel-Crafts conditions at room temperature leads to:
1) Toluene as the only product
2) A mixture of toluene, ortho xylene and para xylene.
3) Only ortho xylene
4) Only meta xylene
Answer: 2
HOMEWORK
1) What is Friedel crafts alkylation? What are its limitations?
2) Why anhydrous AlCl3 is used as a catalyst?
3) What is the product formed in Friedel-crafts alkylation of 1,4-dimethoxybenzene?
4) Write a comment on Friedel crafts alkylation of anisole.
7) What is the electrophile in the alkylation of benzene with methyl iodide?