The Mannich reaction is the aminoalkylation reaction, involving the condensation of an enolizable carbonyl compound (α-CH acidic compound) with a nonenolizable aldehyde (like formaldehyde) and ammonia; or a primary or a secondary amine to furnish a β-aminocarbonyl compound, also known as Mannich base.
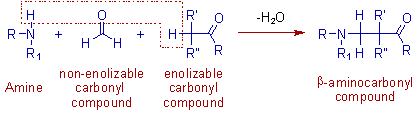
* Instead of formaldehyde, other aliphatic or aromatic aldehydes or ketones can be employed.
* The amine used may be ammonia or 1o or 2o aliphatic amine. Mostly dimethyl amine is used. The aromatic amines do not undergo Mannish reaction.
The reaction is usually carried out with the hydrochloride salt of amine. This salt exists in equilibrium with the free amine and proton. Hence the acidic conditions are maintained in Mannich reaction.
The Eschenmoser's salt, [(CH3)2N=CH2]+I- is used as a source of formaldehyde and dimethyl amine for Mannich reactions.
* The α-CH acidic compounds include carbonyl compounds, nitriles, aliphatic nitro compounds, alkynes, α-alkyl-pyridines or imines, activated phenyl groups and electron-rich heterocycles such as furan, pyrrole, thiophene, Indole etc.
* The reactions are usually carried out in aqueous or alcoholic solutions.
* Since the β-aminocarbonyl compounds can be conveniently reduced to β-aminoalcohols, which show considerable pharmacological activity, the Mannich reaction plays an important role in pharmaceutical chemistry.
MECHANISM OF MANNICH REACTION
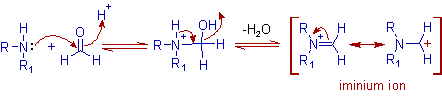
* Initially an iminium ion is formed due to nucleophilic addition of amine to formaldehyde and subsequent loss of water molecule.
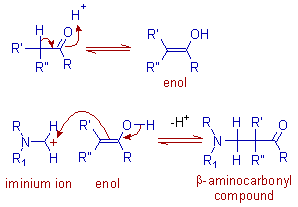
* Since the reaction is carried out in acidic conditions, the enolizable carbonyl compound is converted to enol form, which attacks the iminium ion at positively charged carbon adjacent to nitrogen to give finally a β-aminocarbonyl compound.
ILLUSTRATIONS & APPLICATIONS OF MANNICH REACTION
1) 4-(dimethylamino)butan-2-one is obtained when acetone reacts with formaldehyde and dimethylaminium chloride.
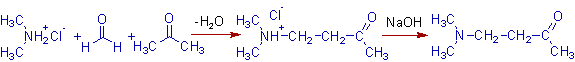
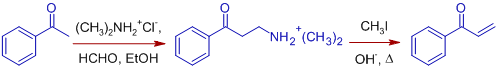
2) The Mannich reaction of acetophenone with formaldehyde and dimethylaminium chloride in alcohol furnishes the salt of 2-(dimethylamino)-1-phenylethanone, which can be conveniently eliminated to acrylophenone (1-phenylprop-2-en-1-one), an α,β-unsaturated compound by converting it into a quaternery salt and subsequent heating (Hoffman elimination).
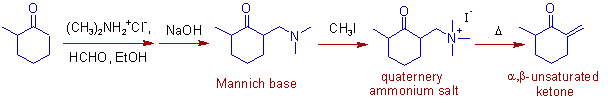
3) In the following example of Mannich reaction, the 2-[(dimethylamino)methyl]-6-methylcyclohexanone, a Mannich base is obtained from the kinetically favored enol form of 2-methylcyclohexanone. Thus formed Mannich base is methylated to give a quaternary ammonium salt which undergoes elimination easily to give an α,β-unsaturated compound (2-methyl-6-methylidenecyclohexanone).
4) The Mannich reaction on Pyrrole with dimethyl amine and formaldehyde is shown below. The use of strong acids is avoided to suppress the unwanted polymerization of pyrrole. Hence acetic acid is used as solvent.
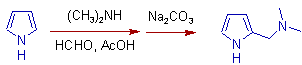
5) Phenol gives a trisubstituted product in the Mannich reaction.
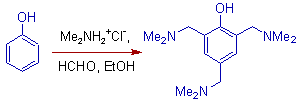
6) Ethylmalonic acid also undergoes aminomethylation at active -CH site as shown below.
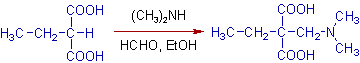
7) Phenylacetylene readily reacts with formaldehyde and dimethylamine to give the following Mannich base.
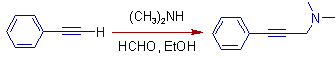
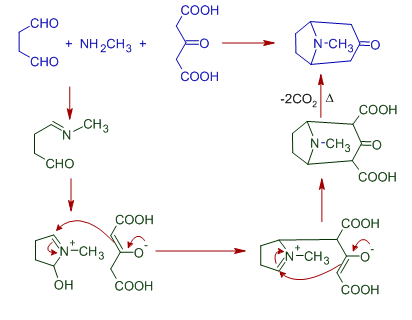
8) The Robinson-Schopf synthesis of Tropinone involves Mannich reaction. The succindialdehyde (butanedial) is treated with methylamine and 3-oxoglutaric acid (3-oxopentanedioic acid) to get Tropinone.