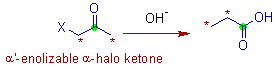
* The Favorskii rearrangement is the base catalyzed rearrangement of enolizable α-haloketones or cyclopropanones to carboxylic acids or their derivatives.
The α-haloketones must contain acidic α'-hydrogens.
* In Favorskii rearrangement, the esters are formed if alkoxides are used as bases. Whereas the amides are formed when amines are used as bases.
* The Favorskii rearrangement of α-halo cyclic ketones results in ring contraction.
MECHANISM OF FAVORSKII REARRANGEMENT
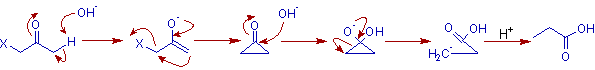
* Initially, the α'-carbon is deprotonated to generate an enolate ion which is followed by intra molecular nucleophilic substitution to give a cyclopropanone by ring closure.
* Thus formed cylcopropanone undergoes nucleophilic addition by a base at the carbonyl carbon which is followed by the cleavage of the CO-Cα bond. Usually the cleavage occurs so as to give less substituted and more stable carbanion.
ILLUSTRATIONS
1) In the following Favorskii rearrangement, 2-Chlorocyclohexanone can be converted into ethyl cyclopentanecarboxylate by treating with sodium ethoxide. The six membered ring is contracted to the five membered one.

2) 3-Bromobutan-2-one yields 2-methypropanoic acid as major product when treated with alkali. The ring opening of cyclopropanone derivative occurs to give less substituted carbanion.
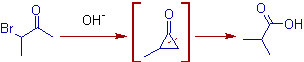
3) The α-haloketones will rearrange to amides when the Favorskii reaction is catalyzed by amines.
4) Synthesis of cubane involves favoroskii rearrangement step as shown below.
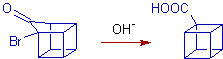
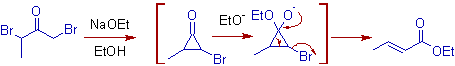
5) α,α-dihaloketones or α,α'-dihaloketones rearrange to give α, β-unsaturated carboxylic acid derivatives under Favorskii conditions. For example, 1,3-Dibromobutan-2-one will give ethyl but-2-enoate when treated with sodium ethoxide.
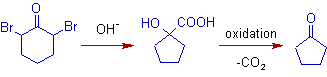
6) The rearrangement of α,α'-dihaloketones to α-hydroxycarboxylic acids, followed by oxidative decarboxylation to the ketones is known as Wallach degradation.
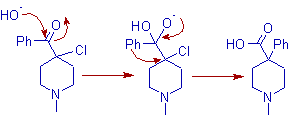
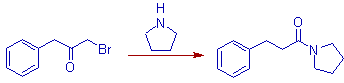
7) The rearrangement of non enolizable α-haloketones in presence of bases is called quasi-Favorskii rearrangement. The synthesis of powerful pain killer Pethidine (also known as Demerol or Meperidine) makes use of this rearrangement.
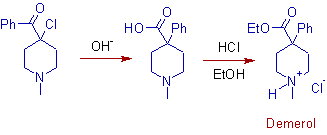
The quasi-Favorskii rearrangement involves no cyclopropanone intermediate and follows a semibenzilic mechanism as shown below.