The parallels between organic and inorganic compounds were first described by Roald Hoffman, a Nobel laureate in chemistry, 1981. He developed the concept of isolobal analogy or principle to show the connection between two important areas of chemistry. According to this concept,
the molecular fragments of a cluster are deemed to be isolobal if they possess same number of frontier molecular orbitals with:
i) similar shape, symmetry and radial extent;
ii) approximately the same energy;
iii) same number of electrons available for cluster bonding.
The isolobal fragments may not be isostructural and isoelectronic. They only possess similar characteristics for the frontier molecular orbitals.
The relation between isolobal fragments is indicated by double headed twirly
arrows i.e., with a tear-drop (![]() ).
).
These isolobal fragments provide electrons for cluster bonding that lead to variety of cluster molecules, at least theoretically.
Examples:
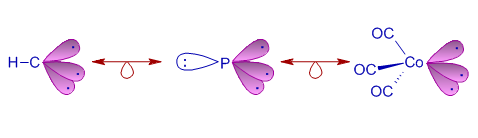
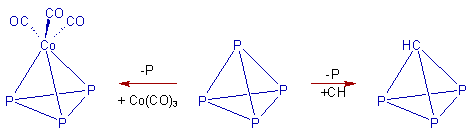
1) The C-H group (carbyne), P atom and Co(CO)3 fragment are isolobal since each of them possesses 3 similar orbitals and can contribute 3 electrons for cluster bonding.
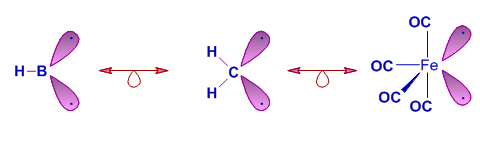
2) The B-H group, the CH2 group and Fe(CO)4 fragment are isolobal. These can contribute 2 electrons for cluster bonding.
Note: The number of skeletal electrons (SE) that can be contributed for cluster bonding by a molecular fragment can be calculated as follows.
SE = C - v + x
Where
v = no. of valence electrons (including s and d orbitals incase of transition elements)
x = no. of electrons contributed by ligand groups
And
C = 8 for p-block elements other than boron group
or 6 for boron group elements
or 18 for transition elements in 18 electron species
or 16 for transition elements in 16 electron species
Starting from methane, a molecule that obeys octet rule, fragments like CH3, CH2, CH can be obtained by homolytic cleavage of a successive C–H bonds as shown below. For example, homolytic fission of a C-H bond in methane generates the methyl radical, which has one frontier orbital with one electron in it.
Whereas, transition metal fragments are generated by removing one L+ from the complex like CrL6 to generate a fragment MnL5. In this case, the Cr metal is replaced by Mn to remove the negative charge (when the proton number increases, the excess negative charge is neutralized).
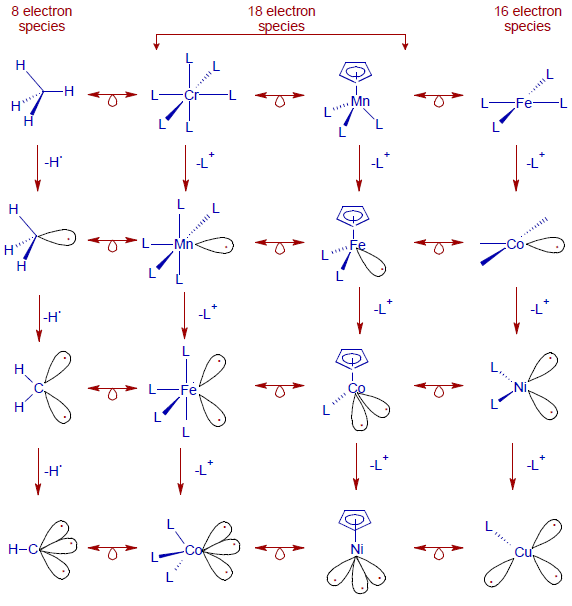
Thus it is possible to draw the following isolobal analogy between metal complexes and organic moeities.
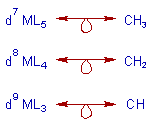
* Theoretically it is possible to substitute a fragment of a molecule by another isolobal fragment to generate a new molecule with very similar bonding.
However there is no guarantee that the “product” of an isolobal transformation is as stable, kinetically or thermodynamically, as the “reactant”.
It may or may not be possible to synthesize the new molecule generated by the isolobal transformation.
E.g. A phosphorus atom in P4 molecule can be replaced by either -CH group or -Co(CO)3.
Author: Aditya vardhan Vutturi