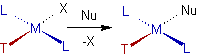
It is observed that during the substitution reactions of square planar metal complexes, some ligands preferentially direct the substitution trans to themselves. i.e., the choice of leaving group is determined by the nature of ligand trans to it.
The Trans effect can be defined as the effect of a ligand over rate of substitution of another ligand positioned trans to it in the square planar complexes.
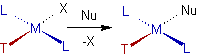
'T' is the trans directing group and 'Nu' is the nucleophilic ligand which preferentially substitutes the ligand 'X' which is trans to ligand 'T'.
* The Trans effect was first recognized by Ilya Ilich Chernyaev, a russian chemist in square planar complexes of Platinum(II).
* However the Trans effect is also observed in octahedral complexes.
* The true Trans effect is a kinetic effect.
In general there are two factors contributing to trans direction of substitution as described below:
1) Trans influence: This is a thermodynamic factor. Some ligands weaken the M-L bond trans to them in the ground state and thus by facilitating the substitution.
E.g. Strong σ- donors like H-, I-, Me-, PR3 etc., destabilize the M-L bond trans to themselves and thus by bringing the easy substitution of that ligand.
2) Trans effect: This is a kinetic factor and considered as true trans effect. It occurs by the stabilization of the transition state.
E.g. The strong π-acceptors like NO+, C2H4, CO, CN- etc., stabilize the transition state by accepting electron density that the incoming nucleophilic ligand donates to the metal through π-interaction.
Note: This is debatable since the π-interaction may increase the strength of M-L bond especially in the trans position.
Kinetic studies: Most of the kinetic work is done on square planar Pt(II) complexes to monitor the trans effect during the substitution reactions. The kinetic studies indicate the associative mechanism as described below.
* Both ΔS‡and ΔV‡ are negative indicating the associative mechanism, either A or Ia.
Where:
ΔS‡ = Entropy of activation
ΔV‡ = Change in volume while forming the transition state from the reactants.
A negative ΔV‡ corresponds to the transition state being compressed relative to the reactants. The volume of transition state is less than the combined volume of the reactants.
* The experimentally observed rate law for a square planar substitution reaction;
ML2TX + Nu ------> ML2TNu + X
can be written as:
rate = k1[ML2TX] + k2[ML2TX][Nu]
Where:
T = trans directing ligand,
X = ligand positioned trans to T.
Nu = Nucleophile
The dependence of rate on the concentration of Nu indicates the associative mechanism.
* The stereo chemical retention of configuration is observed during substitution.
* In some cases the 5 or 6 coordinated species are isolated during the reaction.
* Presence of bulky groups on the metal complexes decrease the rate of substitution. It indicates again the associative mechanism.
Mechanism:
The above kinetic data along with preferential substitution at trans position of certain ligands suggest the possible formation of 5-coordinate trigonal planar transition state (or intermediate).
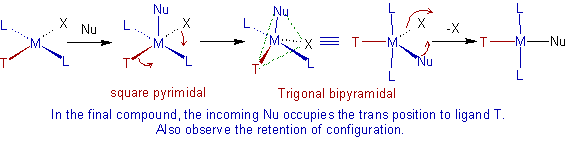
Why the trans substitution is favored?
In the 5-coordinate Trigonal bipyramidal transition state, the electrostatic repulsion is decreased due to removal of electron density in the equatorial plane. The removal of electron density is facilitated by the π-interaction of the trans directing ligand, T as shown below.
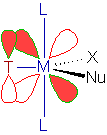
* The square planar substitution reactions occur slowly due to loss of CFSE during the formation of trigonal bipyramidal complex from square planar one. The loss of CFSE is increased down the group. Hence the square planar substitutions of 4d and 5d series are slower. This is why most of the square planar substitution kinetic studies are done on Pt(II) complexes.
* The rough order of trans-directing effect of various ligands is:
NO+, CO, CN-, C2H4 > PR3, H- > Me- > Ph- > NO2-, I-, SCN- > Br- > Cl- > Py, NH3, OH-, H2O
The Trans effect can dictate the product formed in the substitution reactions. The classic example of Trans effect is the synthesis of cis-platin, cis-diamminedichloridoplatinum(II). It is prepared by substituting the two chloro groups of PtCl42- by ammonia molecules.
In the first step, any of the chloro group is substituted by ammonia randomly. But in the second step, the ammonia group preferentially substitutes the chloro group cis to the first ammonia. This can be attributed to the fact that the Cl- has a larger trans effect than NH3.
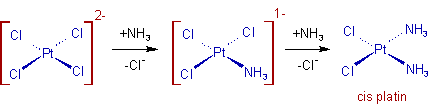
Whereas, the trans product is obtained by starting from Pt(NH3)42+. In this case the second Cl group is substituted preferentially at trans position to the first one.
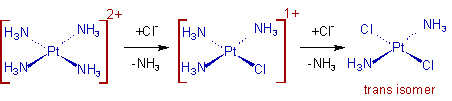
Author: Aditya vardhan Vutturi