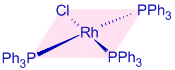
RhCl(PPh3)3 - Chlorotris(triphenylphosphine)rhodium(I), is known as Wilkinson’s catalyst. It is used as a homogeneous hydrogenation catalyst. It is a diamagnetic square planar 16-electron complex. The oxidation state of Rhodium in Wilkinson's catalyst is +1 and it shows dsp2 hybridization for Rhodium.

Preparation:
* It can be prepared by reacting RhCl3.3H2O with excess PPh3 in EtOH.
![]()
Uses:
It is used in the selective hydrogenation of alkenes and alkynes without affecting the functional groups like: C=O, CN, NO2, Aryl, CO2R etc.

MECHANISM OF HYDROGENATION - CATALYTIC CYCLE
Indeed, Wilkinson's catalyst is a pre-catalyst that is converted to an active form by losing one triphenylphosphine ligand before entering the catalytic cycle. Usually, the solvent molecule fills the vacant site.
Initially, the catalyst activates the molecular dihydrogen by oxidative addition mechanism to give a 18 valence electron dihydrido complex. The oxidation state of Rh is increased to +3. Thus formed dihydrido complex binds to the olefin in the next step with the concomitant loss of solvent or PPh3 ligand. Since the activation of dihydrogen occurs before addition of olefin, this path is referred to as dihydride path .
Now one of the hydrogen undergoes migratory insertion at the double bond. This is a slow step i.e., Rate Determining Step (RDS).
Immediately and finally, the alkane is released rapidly by an irreversible reductive elimination step that completes the catalytic cycle. The oxidation state of Rh is decreased to +1 and the catalyst is regenerated.
However, other paths and intermediates are also possible under the given reaction conditions (see below).
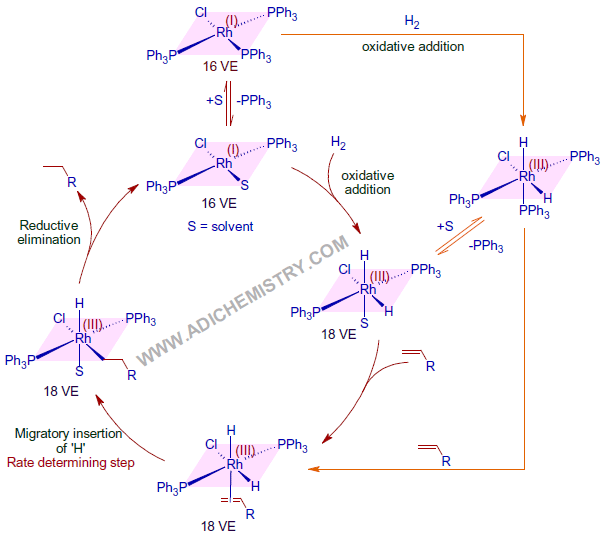
Above mechanism is supported by following observations.
* The rate of reaction decreases when excess of PPh3 is added; indicating the initial dissociation of one of the PPh3 ligand before dihydrogen activation.
* It is observed that strong π-acids like ethylene act as poisons by binding strongly with the electron rich Rh metal center and inhibit hydrogenation.
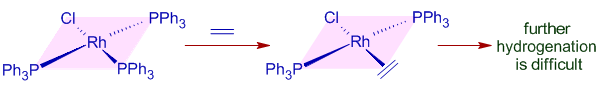
Though ethylene cannot be hydrogenated in presence of Wilkinson's catalyst under normal conditions, hydrogen transfer can be achieved with preformed dihydrido complex.
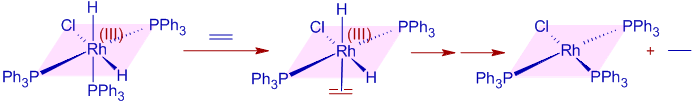
The rates of hydrogenations decrease with increase in the alkyl group substitution on double bond mirroring their relative binding affinities to the metal center. It is also partly due to steric factors.
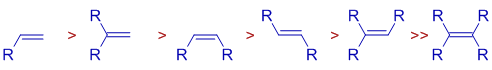
Therefore, for a successful hydrogenation, oxidative addition of dihydrogen prior to the binding of olefin is crucial.
* There is minimal scrambling of H/D in the product, when an equimolar mixture of H2 and D2 are used.
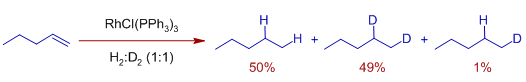
This indicates the formation of dihydrido complex that transfers both of its hydrido ligands to the olefin and that the final reductive elimination step is very fast and irreversible.
SELECTIVE HYDROGENATION BY WILKINSON'S CATALYST
Wilkinson's catalyst can be used to achieve selective hydrogenations.
* Less substituted and sterically less hindered double bonds are selectively hydrogenated.
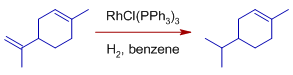
* Exocyclic double bonds are selectively hydrogenated over endocyclic double bonds.
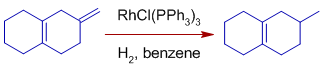
* Cis alkenes are reduced rapidly than trans alkenes.
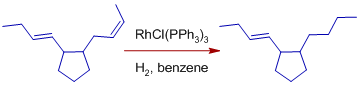
* Isolated double bonds are rapidly hydrogenated over conjugated dienes.

* Terminal alkynes are hydrogenated more rapidly than terminal alkenes. The selectivity can be enhanced by using acidic alcoholic co-solvents.

* Functional groups like C=O, C=N, NO2, Aryl, CO2R etc., are unaffected. The compatibility of Wilkinson's catalyst with polar multiple bonds indicates the metal hydride bond is primarily covalent in character.
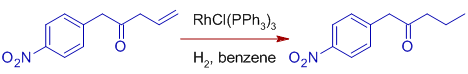
Note: But sterically unhindered aldehyde groups are susceptible to decarbonylation, also rendering the catalyst ineffective.
* Unsaturated substrates containing polar functionality are hydrogenated more rapidly. It may be due to easy coordination of olefin to the catalyst that is assisted by polar functional group.
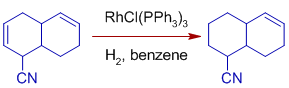
Hydrogenations catalyzed by Wilkinson's catalyst involve stereospecific syn hydrometallation of the multiple bond followed by stereospecific reductive elimination. Hence the hydrogenation of olefins or alkynes result in syn addition products.
E.g. Hydrogenation of Maleic acid or Fumaric acid with D2 in presence of Wilkinson's catalyst is diastereoselective. Hydrogenation of Maleic acid with D2 give meso compound exclusively.
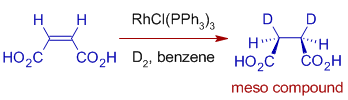
Whereas, with fumaric acid, a racemic mixture is formed.
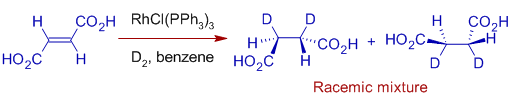
Hence Wilkinson's hydrogenation is both stereospecific as well as stereoselective.
It is stereospecific in the sense that one stereo isomer gives only one stereo isomer as exclusive product. It is due to stereospecific nature of the mechanism.
However it is also stereoselective since only one diastereomer is formed selectively as major product. This is also partly due to other mechanisms operating under reaction conditions.
It is observed that hydrogenation of alkynes give cis alkenes as major products.
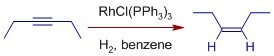
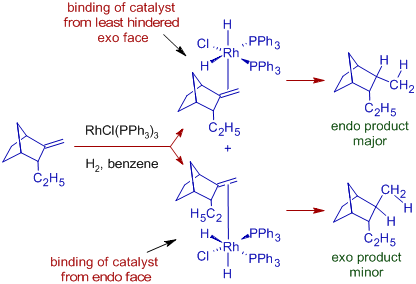
The diastereoselectivity of Wilkinson's hydrogenation is evident in the formation of endo product as major one with the following bicyclic system. The catalyst binds to the double bond from the least hindered exo face of the bicyclic system that is followed by syn addition of two hydrogen atoms.
Since Rh(II) is a low valent and coordinately unsaturated metal and can bind to CO strongly, the Wilkinson's catalyst can be used to bring about decarbonylation of aldehydes through oxidative addition route.
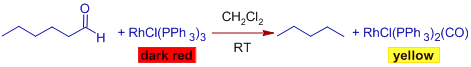
Mechanism:
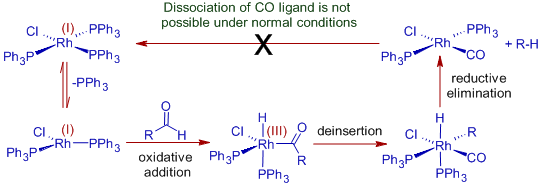
The process is non-catalytic, since the catalyst cannot be regenerated. The complex, chlorocarbonylbis(triphenylphosphine) rhodium, formed during the reaction is quite stable and it is not possible to dissociate CO ligand at mild temperatures. Hence stoichiometric amounts of the complex is required to carry out this conversion.
However, it is possible to make decarbonylation reaction catalytic with Wilkinson's catalyst by employing DiPhenylPhosphoryl Azide (DPPA), which removes CO ligand from chlorocarbonylbis(triphenylphosphine) rhodium to regenerate the active form of catalyst.
PREVIOUS YEAR QUESTIONS WITH KEY & EXPLANATION FOR THIS TOPIC ARE AVAILABLE AT
ADICHEMISTRY CSIR NET, GATE & IIT JAM Chemistry study material
Question-1) The oxidation state of rhodium in wilkinson's catalyst is:
A) +2
B) -1
C) +1
D) +3
Answer: C
Question-2) The coordination number of metal in wilkinson's catalyst is :
A) 4
B) 7
C) 5
D) 6
Answer: A
Question-3) Wilkinson catalyst formula :
A) RhCl(Ph)3
B) RhCl(PPh3)3
C) RhCl(PH3)3
D) RuCl(PPh3)3
Answer: B
Question-4) wilkinson catalyst contains :
A) Ruthenium
B) Cobalt
C) Rhodium
D) Zinc
Answer: C
Question-5) wilkinson's catalyst is used in :
A) Dehydrogenation of aliphatic compounds
B) Hydrogenation of olefins
C) Oxidation of alcohols
D) Reduction of aromatic compounds
Answer: B
Question-6) wilkinson's catalyst is used in :
A) Dehydrogenation of aliphatic compounds
B) Hydrogenation of olefins
C) Oxidation of alcohols
D) Reduction of aromatic compounds
Answer: B