- What are silicates? Formula....?
- Types & Classification of silicates with examples
- Practice questions
What are Silicates? Formula......?
Silicates are the minerals containing silicon and oxygen in tetrahedral SiO44- units, which are linked together in several patterns. Depending on the way the tetrahedral units are linked, the silicates are classified into the following types.
TYPES & CLASSIFICATION OF SILICATES
- Ortho silicates (or Nesosilicates)
- Pyro silicate (or Sorosilicates)
- Cyclic silicates (or Ring silicates)
- Chain silicates (or pyroxenes)
- Double chain silicate (or amphiboles)
- Sheet or phyllosilicates
- Three dimensional (or tecto) silicates
1) ORTHO SILICATES
Ortho silicates (or Neso or Island silicates) are the simplest silicates which contain discrete SiO44- tetrahedral units. The basic structural unit of ortho silicate unit is shown below.
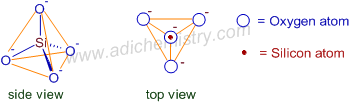
The ortho silicate ion is the strong conjugate base of weak orthosilicic acid as well as it will not persist in aqueous solutions. Hence in nature, ortho silicate minerals are rare and only found with cations which form highly insoluble salts.
Examples of Ortho silicates:
1) Phenacite (also known as phenakite) - Be2SiO4
2) Willemite - Zn2SiO4 - A minor silicate ore of zinc. Highly fluorescent (green) under shortwate UV.
Note: The Be2+ and Zn2+ ions are tetrahedrally surrounded by the oxygen atoms of silicate.
3) Olivine - (Fe/Mg)2SiO4 : Typically green in color. The cations are octahedrally coordinated to the oxygen atoms of the silicate.
4) Zircone - ZrSiO4 : The oldest mineral on Earth. The coordination number of Zr4+ is 8.
2) PYRO SILICATES
Pyro silicate (or Soro silicate or disilicate) contain Si2O76- ions which are formed by joining two tetrahedral SiO44- which share one oxygen atom at one corner (one oxygen is removed while joining). Structure of pyrosilicate is shown below.
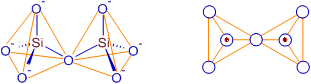
The pyrosilicate ion is less basic than orthosilicate ion. There only one mineral in nature containing pyrosilicate ion.
E.g. 1) Thortveitite - Sc2Si2O7
3) CYCLIC SILICATES
Cyclic silicates contain (SiO3)n2n- ions which are formed by linking three or more tetrahedral SiO44- units cyclically. Each unit shares two oxygen atoms with other units.
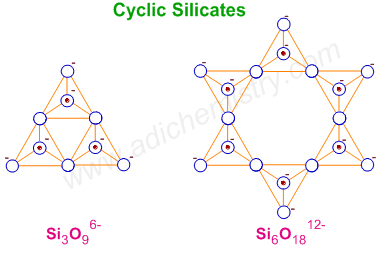
E.g.
1) Benitoite - BaTi(SiO3)3 : containing three tetrahedra arranged cyclically [Si3O9)6-].
2) Beryl - Be3Al2(SiO3)6 : containing six-silicate rings [Si6O18)12-]. It is an aluminosilicate. Each aluminium is surrounded by 6 oxygen atoms octahedrally. Well-known varieties of beryl include emerald and aquamarine.
4) CHAIN SILICATES (PYROXENES)
Chain silicates or pyroxenes contain (SiO3)n2n- ions which are formed by linking ‘n’ number of tetrahedral SiO44- units linearly. Each unit shares two oxygen atoms with other units.
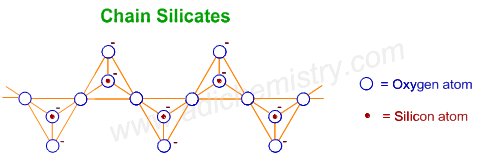
Examples of chain silicates:
1) Spodumene - LiAl(SiO3)2 - a pyroxene mineral consisting of lithium aluminium inosilicate
2) Diopsite - CaMg(SiO3)2
3) Wollastonite - Ca3(SiO3)3
Note: The formula of cyclic silicates as well as chain silicates is (SiO3)n2n-. Hence these are considered as an oligomers of the unknown SiO32- ion.
5) DOUBLE CHAIN SILICATES (AMPHIBOLES)
The general formula of double chain silicates (or Amphiboles) is (Si4O11)n6n- . There are two types of tetrahedra: those sharing 3 vertices and those sharing only 2 vertices.
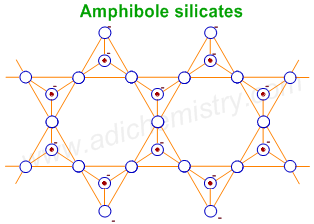
E.g.
1) Asbestos - These are noncombustible fibrous silicates. They have been used for thermal insulation material, brake linings, construction material and filters. These are carcinogenic amphiboles affecting the lungs. Hence their applications are restricted nowadays.
2) Tremolite - Ca2Mg5(Si4O11)2(OH)2
Note: The single and double chain silicates are collectively known as Inosilicates.
6) SHEET SILICATES (PHYLLO SILICATES)
The general formula of Sheet or Phyllo or two dimensional (2-D) silicates is (Si2O5)n2n- . Each SiO4 tetrahedron shares three oxygen atoms with others and thus by forming two-dimensional sheets. These silicates can be cleaved easily just like graphite. The layers are held together by weak van der Waal's forces.
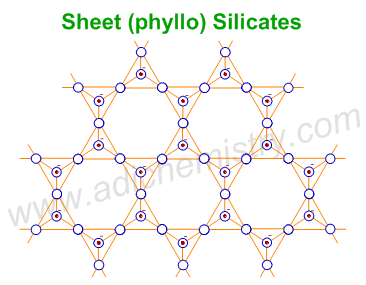
E.g.
1) Talc - Mg3Si4O10(OH)2 : It is the main ingredient of soap stone. It is the softest material with a smooth and greasy touch.
2) Micas : General formula is X2Y4–6Z8O20(OH,F)4
Where X = K, Na, or Ca
Y = Al, Mg, or Fe
Z = Si or Al
E.g. Muscovite mica - KAl2(AlSi3O10)(F,OH)2 - split into thin layers extremely easily
and Lepidolite - KLi2Al(Al,Si)3O10(F,OH)2
3) Clay : It is an aluminosilicate with sheet structure.
4) Kaolinite - AlSiO5(OH)5
7) THREE DIMENSIONAL SILICATES (TECTO SILICATES)
The general formula of three dimensional (3-D) or tecto or Framework silicates is (SiO2)n . All the oxygen atoms of SiO4 are shared with other tetrahedra and thus by forming three-dimensional network.
E.g. SiO2 - Quartz, Tridymite and Crystobalite - These are the crystalline forms of silica.
When SiO44- units are replaced by AlO45- units, three dimensional aluminosilicates are formed.
E.g. Feldspar, Zeolites, Ult etc.,
A FEW IMPORTANT POINTS ABOUT SILICATES
* Silicate minerals are very common in the Earth crust since Oxygen and Silicon are the most abundant elements.
* The degree of polymerization is denoted by Oxygen to Silicon ratio (O/Si). Greater the degree of polymerization, lower will be the O/Si ratio. The values of O/Si for ortho silicates (lease polymerized) = 4:1 while for tecto silicates (most polymerized silicate) = 2:1.
* With increase in the degree of polymerization, there is decrease in the charge per silicon atom as well as the basicity of silicate mineral. Indeed, silica (SiO2) is an acidic oxide.
* The basic silicate minerals readily react with weak acids and undergo weathering.
SILICATES - PRACTICE MCQ QUESTIONS & ANSWERS CSIR NET GATE IIT JAM JEE NEET
Question-1) Which statement about silicates is NOT correct?
a) The basic unit of silicates is SiO44-. In this the silicon is 4 - coordinated to oxygen atoms tetrahedrally.
b) Asbestos is an amphibole silicate.
c) Talc and mica are examples of silicates with chain structures.
d) Zeolites are aluminosilicates.
e) Some silicates contain discrete molecular ions.
Hint: Talc and mica are sheet or phyllo silicates.
Question-2) (SiO3)n2n- may be the empirical formula of:
a) Cyclic silicates
b) Pyro silicates
c) Chain silicates
d) both a & c
Question-3) The general formula of silicate ion present cyclic silicates is:
a) SiO44-
b) Si2O52-
c) Si2O76-
d) (SiO3)n2n-
Question-4) Which of the following statement is NOT true?
a) Clay is an alumino silicate with sheet structure.
b) In three dimensional aluminosilicates, the SiO44- is replaced by AlO45- units.
c) In tecto silicates, all the oxygen atoms in SiO4 tetrahedra are shared.
d) Quartz is a phyllosilicate.
Question-5) Layered silicate structures in clays consists the following group.
a) SiO44-
b) Si2O52-
c) Si2O76-
d) (SiO3)n2n-
Question-6) Amphibole silicate minerals consist of
a) single silicon tetrahedra
b) chains of silica tetrahedra
c) double chains of silica tetrahedra
d) sheets of silica tetrahedra
Question-7) Which of the following is correct about the ion exchange on aluminosilicates?
a) Na+ is sorbed more strongly than K+
b) Ca2+ is sorbed less strongly than Na+
c) NO3- is sorbed more strongly than Na+
d) Al3+ is sorbed more strongly than Mg2+
Explanation: Greater the charge density greater is the sorption. And you know charge density is inversely related to the radius. Greater the charge and smaller the radius, higher is the charge density.
Question-8) Beryl, Be3Al2Si6O18 is:
a) an orthosilicate
b) a pyrosilicate
c) a cyclic silicate
d) None
Not a Hint!: Emerald and aquamarine are the gem forms of beryl.
Question-9) Which of the following statement is/are correct for amphibole silicates?
a) The empirical formula is [(SiO3)2-]n
b) There are two types of tetrahedra: those sharing 3 vertices & those sharing 2.
c) There are only one type of tetrahedra.
d) The empirical formula is [(Si4O11)6-]n
Question-10) The formula of the pyrosilicate ion is:
a) SiO44-
b) Si3O92-
c) Si2O76-
d) Si6O1812-
Question-11) In normal silicates, each silicon atom is surrounded by
a) five oxygen atoms in a trigonal bipyramidal geometry
b) four oxygen atoms in a square planar geometry
c) four oxygen atoms in tetrahedral geometry
d) six oxygen atoms in octahedral geometry
Question-12) Which of the following is an example of cyclic silicate?
a) Spodumene
b) Olivine
c) Beryl
d) Asbestos
Question-13) The empirical formula of double chain silicates is:
a) SiO44-
b) Si3O92-
c) Si2O76-
d) (Si4O11)n6n-
Question-14) The silicates in which the three oxygens atoms at the vertices of tetrahedra are shared are:
a) Amphiboles
b) pyroxenes
c) phyllo silicates
d) pyrosilicates
Question-15) The ratio of ‘Si’ to ‘O’ in phyllosilicates is:
a) 2 : 4
b) 1 : 4
c) 2 : 5
d) 1 : 5
16) Which of the following mineral of lithium has pyroxene type silicate structure?
a) Lepidolite
b) Spodumene
c) Petalite
d) None
Note: Petalite, LiAlSi4O10 is a tecto silicate.
17) The formula of cyclic silicate in which 6 tetrahedra are arranged cyclically is:
a) Si6O1812-
b) Si6O186-
c) Si6O62-
d) Si6O186-
18) In which of the following silicates, there are two types of SiO4 tetrahedra: those sharing 3 vertices & those sharing 2.
a) Pyroxenes
b) Phyllo silicates
c) Amphiboles
d) Tecto silicates
19) The number of oxygen atoms involved in sharing in [Si3O9]6- ion is:
a) 2
b) 3
c) 6
d) 4
20) Among feldspar, muscovite mica and zeolite,
a) all are three dimensional silicates
b) feldspar and zeolite are three dimensional, while muscovite mica is layered
c) feldspar is three dimensional, while zeolite and muscovite mica are layered
d) all are layered silicates
21) The overall charge present on the cyclic silicate anion [Si6O18]n- is:
a) 24
b) 12
c) 18
d) 6
22) The silicate mineral with Lower degree of polymerization among the following is:
1) Grunerite - Fe7Si8O22(OH)2
2) Pyrophyllite, Al2Si4O10(OH)2
3) Talc - Mg3Si4O10(OH)2
4) Tremolite - Ca2Mg5(Si4O11)2(OH)2
Hint: Greater the O/Si ratio lower is the degree of polymerization.
23) Which of the following is not an orthosilicate?
a) Phenacite
b) olivine
c) Olivine
d) Beryl
24) BaTi[Si3O9] is a class of:
a) orthosilicate
b) cyclic silicate
c) chain silicate
d) sheet silicate
25) Which of the following silicates have 3D structure?
1)Neso
2)Phyllo
3)Soro
4)Tecto
26) The value of n in the molecular formula BenAl2Si6O18 is:
a) 3
b) 4
c) 6
d) 8
27) The basic structural unit of feldspar zeolite mica and asbestos is :
a) SiO44-
b) SiO2
c) (R2SiO)n
d) (SiO3)n2n-
28) Thortvetite, Sc2Si2O7 is:
a) a pyrosilicate
b) an orthosilicate
c) a sheet silicate
d) an amphibole
29) Which of the following is incorrect about anionic unit of pyrosilicate?
a) It has one oxygen shared between two SiO4 tetrahedra.
b) It has three SiO4 tetrahedra connected cyclically.
c) It has basic nature and hence reacts with weak acids readily.
d) The O/Si ratio is 3.5:1.
30) Silicate having one monovalent corner oxygen atom in each tetrahedron unit is:
a) Cyclic silicate.
b) Phyllosilicate.
c) Amphiboles.
d) Ortho silicates.
31) What type of silicate minerals is shown from this arrangement of silicate tetrahedra?
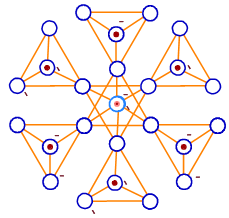
a) Ortho silicate.
b) Cyclic silicate.
c) Framework silicate.
d) Phyllo silicate.
32) Which of the following silicates is referred to as pyroxenes?
a) Single chain silicates.
b) Phyllosilicate.
c) Tectosilicates.
d) Double chain silicates.
33) The mica minerals like Muscovite, phlogopite and Biotite make some rocks to sparkle as they break very easily into thin parallel sheets of flat surfaces , which reflect the light. The reason is:
1) These are ortho silicates containing discrete units of SiO42- tetrahedra.
2) These are framework silicates.
3) These are phyllo silicates with layers which are held together by strong van der Waal's forces.
4) These are phyllo silicates with layers which are held together by weak van der Waal's forces.
34) The Si4+ units in Feldspars are replaced by:
1) Ca2+
2) Na+
3) Al3+
4) K+
35) Name of the structure of silicates in which three oxygen atoms of SiO44- are shared is :
1) Pyrosilicates
2) Pyroxenes
3) Tectosilicates
4) Amphiboles
36) The type of silicate unit present in beryl silicate Be3Al2[Si6O18] is :
1) sorosilicate
2) cyclosilicate with six units
3) sheet silicate
4) cyclosilicate with three units
37) How many oxygen atoms of SiO44- units are shared in the continuous 3d frame work of silicates?
1) 2
2) 3
3) 4
4) 1
38) Beryl is a silicate. Its anion part contains [SinO3n]12-, then the value of n is :
1) 3
2) 6
3) 9
4) 18
39) Name the type of the structure of silicate in which one oxygen atom of SiO44- is shared :
1) Nesosilicate
2) Sorosilicate
3) Phyllosilicate
4) Tectosilicate
40) Silica is a network solid with Si−O−Si bonds. In many aluminosilicates, a few Si atoms in the silica structure are replaced by Al atoms. Zeolites, clays, asbestos etc. are examples of aluminosilicates having different structures. The statement which is not correct is: (NEST 2017)
(A) The basic structural unit in silicates is tetrahedral (SiO4)4−.
(B) Replacement of a Si atom in silica by an Al atom results in negative charge on the Al atom.
(C) Silica dissolves in aqueous NaOH to form sodium silicate in which Na+ is bonded to oxyanions.
(D) Silicones are examples of silicates.
41) The most likely geometry for a silicate unit is: (NEST 2012)
(A) square planar.
(B) square pyramidal.
(C) octahedral.
(D) tetrahedral
42) Egyptian blue CaCuSi4O10 is an example of:
A) sheet silicate
B) Cyclic silicate
C) Pyrosilicate
D) Chain silicate
43) The most used acid catalyst in oil industry and the relevant process are respectively: (CSIR NET JUNE 2012 Solved - AdiChemistry)
1) Aluminophosphate and reforming
2) Aluminosilicate and cracking
3) Aluminosilicate and reforming
4) Aluminophosphate and cracking.
44) The species that result by replacing one quarter of Si(IV) in pyrophyllite [Al2(OH)2Si4O10] with Al(III) Charge balance by K(I)] is (CSIR NET JUNE 2019 Solved - AdiChemistry)
(1) Muscovite
(2) Phlogopite
(3) Montmorillonite
(4) Talc
Question-45) silicates with continuous 3d framework are known as:
(1) Tectosilicates
(2) amphiboles
(3) Pyroxenes
(4) Cyclic silicates
Answer: 1