a) 19.2 x 10-2 m
b) 5.76 x 10-2 m
c) 1.92 x 10-2 m
d) 3.84 x 10-2 m
Answer with Logic:
According to Heisenberg's uncertainty principle, the product of uncertainties in position (Δx) and velocity (Δv) is always equal to or greater than h/nπ.
i.e.
![]()
What is the meaning of "accurate up to 0.001%"?
It indicates that the uncertainty in velocity is 0.001% of actual value, 300 m s-1.
i.e.
uncertainty in velocity (Δv) = 300 x 0.001 / 100 = 3 x 10-3 m s-1
The uncertainty in position (Δx) can be calculated as follows:
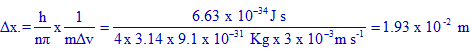
Note: n = 4 for electron.
Conclusion:
The correct option is "c".
(EAMCET 2011-E)
a) 1000 :1
b) 10,000:1
c) 1: 1000
d) 1:10,000
Logic & solution:
The uncertainty in position, Δx is given by the expression:
![]()
The ratio of uncertainties in positions for A and B is:
![]()
Note: h/nπ is constant and Δv values are same.
Conclusion:
Correct option is "c".
(EAMCET 2006-E)
a) 2
b) 0.25
c) 4
d) 1
Logic & solution:
The uncertainty in position, Δx is given by the expression:
![]()
The ratio of uncertainties in positions for A and B is:
![]()
Note: mB = 5mA
Conclusion:
Correct option is "a".
(AIEEE 2002)
a) 2.1 x 10-28
b) 2.1 x 10-34
c) 0.5 x 10-34
d) 5.0 x 10-24
Logic & solution:
The uncertainty in velocity, Δv is given by the expression:
![]()
Conclusion:
Correct option is "a".
(AIEEE 2009)
1) 1.52 x 10-4 m
2) 5.10 x 10-3 m
3) 1.92 x 10-3 m
4) 3.84 x 10-3 m
Logic & solution:
Since the accuracy in velocity is 0.005%, uncertainty in velocity, Δv = 600 x 0.005 / 100 = 3 x 10-2 m s-1.
Uncertainty in position will be:
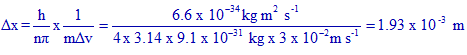
Conclusion:
Correct option is "3".
1) State Heisenberg's uncertainty principle.
2) What will be the uncertainty in momentum of electron if the uncertainty in its position is zero?
Jump to De broglie Wavelength - IIT JEE - NEET - IT JAM solved problems