| Next question > |
(IIT JEE 1983)
a) 2-butene
b) 2-butyne
c) 2-butanol
d) butanal
Logic:
The compounds with each doubly bonded carbon attached to two different groups (like Cab=Cab, Cab=Ccd) exhibit geometrical isomerism i.e., cis and trans forms. The geometrical isomerism arises due to restricted rotation of double bond.
However, even though there is restricted rotation for triple bond, alkynes do not exhibit geometrical isomerism, since the triply bonded carbons are attached to one group each only.
Conclusion:
Option 'a' is correct.
(IIT JEE 1987)
a) 4
b) 5
c) 6
d) 7
Solution:
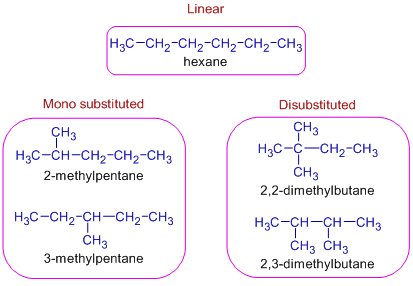
C6H14 indicates saturated hydrocarbon i.e., an alkane. There are 5 chain isomers possible with this formula i.e., one linear, two monosubstituted, two distubstituted.

Conclusion:
Option 'b' is correct.
(IIT JEE 1992)
a) conformers
b) diastereomers
c) enantiomers
d) positional isomers
Answer:
Conformers.
(IIT JEE 1986)
a) methanol
b) diethyl ether
c) acetone
d) dimethyl ether
Logic:
Isomers have same molecular formula but differ in their structures or in spatial arrangement.
Solution:
| Compound | Structural formula | Molecular formula |
| Ethanol | C2H5OH | C2H6O |
| Methanol | CH3OH | CH4O |
| Diethyl ether | C2H5OC2H5 | C4H10O |
| Dimethyl ether | CH3OCH3 | C2H6O |
Only dimethyl ether has same molecular formula as that of ethanol.
Conclusion:
Correct option is: 'd'
(IIT JEE 1997)
a) 1
b) 2
c) 3
d) 4
Logic:
* Theoretically, the total number of enantiomers possible for a molecule is equal to 2n, where n = number of chiral centers.
* However, to show optical activity, the molecule must be asymmetric. Hence some of the configurations may be optically inactive due to symmetry.
Solution:
Butane-2,3-diol, CH3-CH(OH)-CH(OH)-CH3 has two chiral centers. Hence, theoretically, there must be 2n = 22 = 4 enantiomers possible. However, one of the configuration has plane of symmetry and hence is optically inactive. It is called meso isomer. It has no enantiomer. Therefore only three stereoisomers are possible among which only are two optically active.
1) What is stereoisomerism?
2) What are enantiomers?
3) What is the nature of
| Next question > |
Author: Aditya vardhan Vutturi