Lassaigne’s test is used for the detection of elements: Nitrogen (N), Sulfur (S), Chlorine (Cl), Bromine (Br) and Iodine (I). This test involves following two steps.
i) preparation of sodium fusion extract (SFE).
ii) Detection of elements using SFE.
A small amount of organic substance is fused with a small quantity of sodium metal in a fusion tube. The red hot fusion tube is then plunged into distilled water and the contents are boiled for a few minutes, then cooled and filtered. then cooled and filtered.
The filtrate obtained is called sodium fusion extract (SFE) or Lassaigne’s extract. It is usually alkaline. If it is not alkaline, a few drops of NaOH solution may be added to make it alkaline.
Question: Why is sodium fusion extract alkaline?
Answer: The excess of sodium reacts with water to furnsish hydroxide ions along with the liberation of dihydrogen gas.
ii) Detection of elements using SFE
Thus obtained SFE is used to detect the presence of elements like N,S,Cl, Br & I.
The elements in the organic compound react with sodium during fusion reaction as follows:
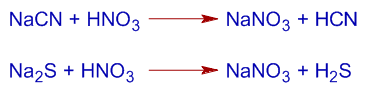
Na + C + N ------------> NaCN (if N is present)
2Na + S ----------------> Na2S (if S is present)
Na + S + C + N --------> NaSCN (if both N & S are present & insufficient amount of Na is used)
Na + X -------------------> NaX (If halogens are present)
Where
X = Cl/Br/I
Hence SFE may contain any of or all of ionic forms of respective elements.
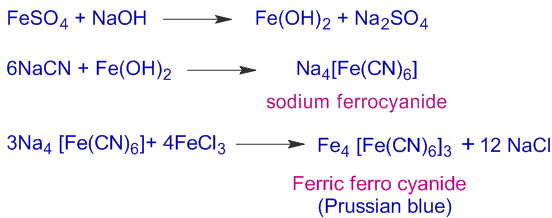
To a portion of SFE, freshly prepared ferrous sulphate, FeSO4 solution is added and warmed. Then about 2 to 3 drops of FeCl3 solution are added and acidified with conc. HCl. The appearance of a Prussian blue color indicates the presence of nitrogen.

Note:
1) In Lassaigne's test, afresh solution of fresh solution of FeSO4 must be used. Otherwise, it is oxidized to ferric sulfate due to aerial oxidation.
2) HCl is added to convert Ferrous hydroxide, a green precipitate to ferrous chloride, which is soluble in water. Otherwise the green precipitate may interfere with Prussian blue color.
Fe(OH)2 + 2HCl --------------> FeCl2 + 2H2O
When both N & S are present:
However if both N & S are present in the organic compound and SFE is prepared with insufficient amount of Na metal, the thiocyanate (SCN-) is formed instead of cyanide ion (CN-). The thiocyanate, SCN- reacts with Fe3+ to give a blood red coloration due to formation of [Fe(SCN)]2+.
Fe3+ + SCN– -------------> [Fe(SCN)]2+
Note: However, if sodium fusion is carried out with excess of sodium, the thiocyanate, SCN- is decomposed to give CN– & S2–. Hence in this case, sulfur and nitrogen are to be identified in separate tests.
i) The appearance of a deep violet color upon addition of a few drops of sodium nitroprusside to sodium fusion extract (SFE) indicates the presence of sulfur.
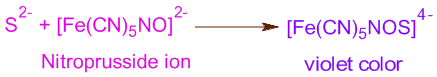
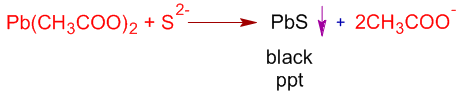
ii) Sulphur can also be detected by adding lead acetate to SFE, acidified with acetic acid. The formation of a black precipitate (PbS) indicates the presence of sulfur.
The sodium fusion extract, SFE can be used to detect the presence of chlorine, bromine and iodine but not fluorine. To detect their presence, the SFE is first acidified with HNO3 and then added with AgNO3 solution.
i) The formation of a curdy white precipitate that is soluble in NH4OH indicates the presence of chlorine in the organic compound.
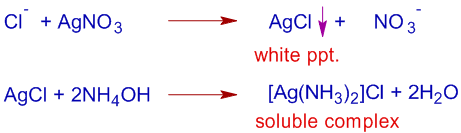
ii) The formation of a pale yellow precipitate that is partially soluble in NH4OH confirms the presence of bromine.
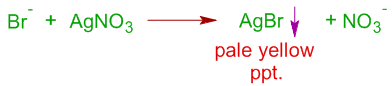
iii) Where as the formation of a yellow precipitate insoluble in NH4OH confirms the presence of iodine in the organic compound.

Note:
1) It is not possible to detect the presence of fluorine since the solubility of AgF is more and thus no precipitate is formed.
2) If nitrogen or sulfur are present in the organic compound, the formation of black precipitates of AgCN or AgS may interfere during the test for halogens. Hence the CN- and S2- have to be removed from the SFE. This is done by boiling the SFE with conc. HNO3 or glacial acetic acid to almost dryness. The CN- and S2- ions are removed as HCN and H2S gases.
| Test | Observation | Inference |
| SFE + FeSO4 + FeCl3 + HCl | i) A prussian blue color is
formed. ii) Blood red coloration is observed. |
i) Nitrogen is confirmed. ii) Both nitrogen and sulfur are confirmed. |
| i) SFE + Sodium nitroprusside ii) SFE + CH3COOH + Pb(CH3COO)2 |
i) A violet coloration is
observed. ii) A black precipitate is formed. |
Sulfur is confirmed. |
| SFE + HNO3 + AgNO3 | i) A white ppt. soluble in NH4OH is formed. ii) A pale yellow ppt. partially soluble in NH4OH is formed. iii) A yellow ppt. insoluble in NH4OH is formed. |
i) Chlorine is confirmed. ii) Bromine is confirmed. iii) Iodine is confirmed. |
Question-1) Which metal is used in Lassaigne's test?
a) K
b) Zn
c) Na
d) Mg
Note: High or low reactivity is not expected.
Answer: c
Question-2) In Lassaigne's test the sodium extract of an organic compound is:
a) Neutral
b) Acidic
c) Alkaline
d) Both alkaline and acidic
Hint: The unreacted sodium reacts with water to give NaOH.
Answer: c
Question-3) Determination of the chlorine is NOT possible without preparing sodium extract in case of:
a) 2,4,6-Trinitrochlorobenzene
b) Benzyl chloride
c) Allyl chloride
d) Tropilium chloride
Answer: d
Note: Few chloro organic compounds give white precipitate when treated with silver nitrate. It is possible due to ionization of the compound by forming a stable carbocation and chloride ion. If the carbocation is not stable, then no ionization is possible. For example, tropilium cation is very stable due to aromaticity.
Question-4) Sodium nitroprusside test for detection of sulphur in organic compound results in formation of deep violet colored solution. This color is due to formation of :
or
The violet colour obtained during the test of sulphur due to formation of:
a) [Fe(CN)5NO]2-
b) [Fe(CN)5NO]4-
c) [Fe(CN)5NOS]4-
d) [Fe(SCN)]2+
Note: For detection of sulphur in organic compounds, sodium nitroprusside is used. It reacts with sulfide ion to give deep violet colored complex.
Answer: c
Question-5) Lassaigne's test for nitrogen will fail in the case of:
or
Which of the following compounds does not perform lassaigne’s test?
a) Urea - NH2CONH2
b) Semicarbazide - NH2CONHNH2
c) Hydrazine - NH2NH2
d) Acetonitrile - CH3CN
Hint: Nitrogen is detected only when cyanide ion is formed during sodium fusion test. It requires carbon along with nitrogen in the organic compound.
Answer: c
Question-6) Lassaigne's test is used for detection of
a) Sulfur
b) Nitrogen
c) Halogens
d) All of the above
Answer: d
Question-7) Sodium nitroprusside is used to confirm the presence of which element in organic compound?
a) Nitrogen
b) Sulfur
c) Carbon
d) Iodine
Answer: b
Question-8)Which complex is formed during nitrogen sulphur test to give blood red colour?
1) [Fe(SCN)]2+
2) [Fe(CNS)]3+
3) [Co(SCN)]2+
4) [Fe(SCN)]+
Answer: 1
Question-9) In Lassaigne's test, the organic compound is first heated with sodium(Na) metal. The Na metal is used because
(a) the melting point of Na metal is low
(b) sodium metal reacts with elements present in organic compounds to form inorganic compounds
(c) Sodium metal reacts with organic compound by giving soluble sodium salts.
(d) sodium is highly reactive metal
Answer: c
Author: Aditya vardhan Vutturi