* The Arndt-Eistert reaction is employed in converting a carboxylic acid to a higher homologue with one additional carbon atom.
| SOCl2 | CH2N2 | Ag+ | ||||
| R-COOH | -------------> | R-COCl | --------------> | R-COCHN2 | ----------------------> | R-CH2CONu |
| ether, Et3N | Nucleophile (Nu) |
* The following steps are involved in the Arndt-Eistert reaction.
1) Conversion of carboxylic acid to an active compound like acid chloride or an anhydride.
R-COOH + SOCl2 --------> R-COCl + SO2 + HCl
2) Conversion of acid chloride to a diazoketone. A base like Et3N is employed to neutralize HCl liberated in this step.
R-COCl + CH2N2 --------> R-COCHN2 + HCl
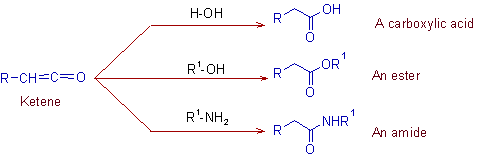
3) The Wolff rearrangement of diazoketone into a ketene and subsequent conversion of it to a higher carboxylic acid or its derivative by using a nucleophile.
| Ag2O, Δ or hν, -N2 | Nucleophile (Nu) | |||
| R-COCHN2 | -------------------------------------> | R-CH=C=O | --------------------------------> | R-CH2CONu |
| diazoketone | Wolff rearrangement | ketene | higher homologue |
* Nucleophile (Nu) = water or alcohol or amines
MECHANISM OF ARNDT-EISTERT REACTION
1) Initially the diazomethane is acylated by the acid chloride to give a diazoketone.
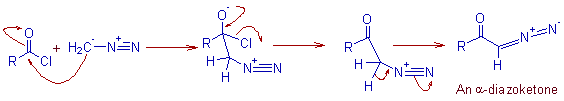
* The HCl liberated in the first step must be neutralized by a suitable base to avoid the formation of chloromethyl ketone. Usually two moles of diazomethane are used to avoid this side reaction.
2) Thus formed diazoketone is rearranged to a ketene. This is called Wolff-rearrangement.
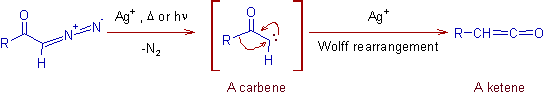
* Silver salts like PhCO2Ag, Ag2O along with heat or light catalyze the Wolff rearrangement.
* The configuration of 'R' group during Wolff rearrangement is retained.
3) The ketene is immediately attacked by an appropriate nucleophile in the solution.
ILLUSTRATIONS
1) Arndt-Eistert reaction is used in the conversion of 4-nitrobenzoic acid to (4-nitrophenyl)acetic acid as shown below.
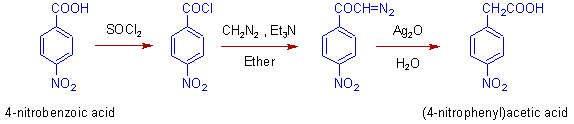
* The nitro group is not affected in the above reaction.
2)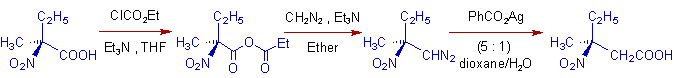
* The carboxylic group is first converted to an anhydride using ethyl chloroformate, ClCO2Et.
* Also note that the configuration at stereogenic carbon is retained.