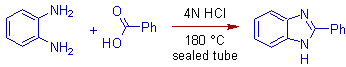* The Phillips reaction involves the condensation of ortho phenylenediamines with organic acids in presence of dilute mineral acids to furnish benzimidazoles.
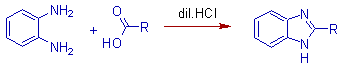
* This method has the advantage that the benzimidazoles, which cannot be prepared by heating the components together, are obtained easily by using dilute acids at lower temperatures.
* Good yields are obtained with aliphatic acids.
* However the yield can be improved by carrying out the Phillips reaction in sealed tubes with aromatic acids also.
MECHANISM OF PHILLIPS REACTION
* Initially one of the amine group is acylated with the organic acid in presence of mineral acid to furnish an N-acylated compound. In the next step, the other nitrogen is also acylated by making bond with the carbonyl carbon of the first acyl group leading to ring closure.
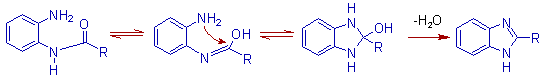
ILLUSTRATIONS OF PHILLIPS REACTION
1) Benzene-1,2-diamine can be condensed with acetic acid in presence of 4N HCl to give 2-methyl-1H-benzimidazole.
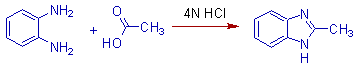
2) 2-phenyl-1H-benzimidazole can be prepared by carrying out the Phillips reaction in a sealed tube at 180oC