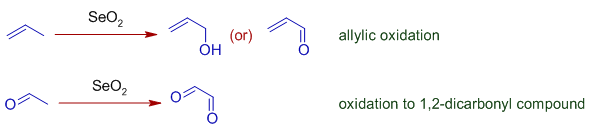
* Selenium dioxide, SeO2 is an oxidizing agent generally employed in the allylic oxidation of alkenes to furnish allylic alcohols, which may be further oxidized to conjugated aldehydes or ketones. It is also used to oxidize the α-methylene group adjacent to a carbonyl group to give a 1,2-dicarbonyl compound. However selenium dioxide can perform several common types of oxidations, such as alcohols to ketones or aldehydes. The oxidations of methylene groups using Selenium dioxide are referred to as Riley oxidations.
Note: SeO2 is sometimes referred to as selenium(IV) oxide.
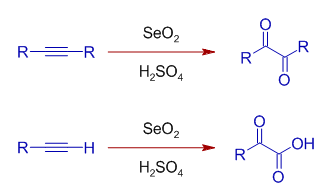
Selenium oxide can also be used to oxidize alkynes in presence of acids. The internal alkynes are converted to 1,2-dicarbonyl compounds, whereas terminal alkynes are oxidized to glyoxylic acids.
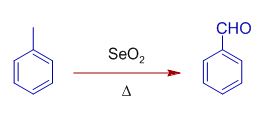
It also oxidizes benzylic methylene, CH2 group to C=O.
Structure & Properties of reagent; Reaction conditions & Workup
Structure & Properties:
* Selenium dioxide is a colorless solid. It exists as one dimensional polymeric chain with alternating selenium and oxygen atoms.
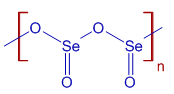
* It sublimes readily and hence the commercial samples of SeO2 can be purified by sublimation.
* SeO2 is an acidic oxide and dissolves in water to form selenous acid, H2SeO3.
Reaction conditions:
* Compounds of selenium are very poisonous and smelly. Hence the reaction setup must be maintained under a fuming cupboard. Variety of solvents can be employed.
* Use of acetic acid as solvent stops the reaction at allylic alcohol stage due to formation of acetate esters.
* A convenient way to carry out the reaction is to use only a catalytic amount of SeO2 along with an oxidizing agent like t-butyl hydroperoxide, that reoxidizes the selenium(II) compounds after each cycle of the reaction. This eliminates the need to get rid of large amounts of selenium compounds, which are toxic and usually smelly. It also ensures the principal product is allylic alcohol by reducing the chances of further oxidation to conjugated carbonyl compounds.
Workup:
* The final workup involves precipitation of selenium or selenium compounds, which can be filtered off before isolation of product from the reaction mixture.
MECHANISM
Allylic oxidation
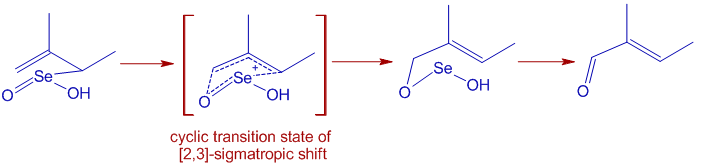
* Selenium dioxide oxidizes allylic positions to alcohol or carbonyl groups. It starts with Alder-ene like 4+2 cycloaddition of of SeO2 to give an allylic selenic acid that further undergoes [2,3]-sigmatropic rearrangement to give an unstable compound that may decompose to allylic alcohol or an allylic carbonyl compound as shown below.
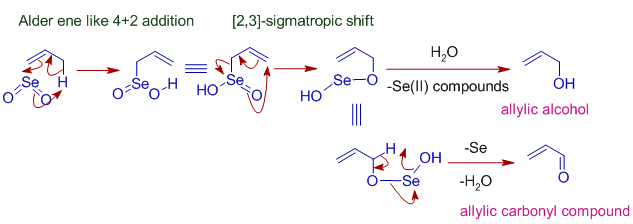
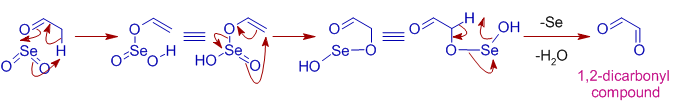
Formation of 1,2-dicarbonyl compounds
* SeO2 can also oxidize α-methylene group on a carbonyl compound to furnish 1,2-dicarbonyl compound. The mechanism involves steps similar to allylic oxidation.
APPLICATIONS
* Catalytic amount of selenium dioxide and t-BuOOH can be employed in the allylic oxidation of cyclohexene to cyclohex-2-en-1-ol, an allylic alcohol.

* Trisubstituted alkenes are oxidized selectively at more substituted end of double bond by giving E-allylic alcohols or conjugated carbonyl compounds predominantly.
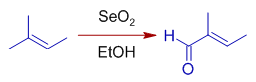
It is because the initial ene type 4+2 cycloaddition involves preferential attack of the more nucleophilic end of double bond at selenium. In this step, alkene uses the π-HOMO to attack the π*-LUMO of Se=O. Meanwhile the π-HOMO of Se=O attacks the σ*-LUMO of C-H of the allylic system.
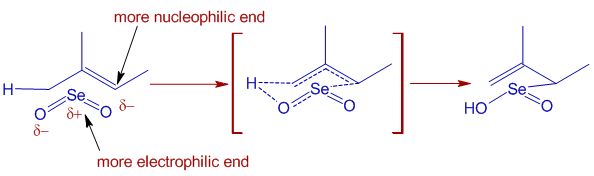
The E-selectivity is due to cyclic nature of final [2,3] sigmatropic step in which the alkyl substituent adopts pseudoequatorial position.
* The allylic oxidation occurs predominantly at most nucleophilic double bond. In the following example, no allylic positions of double bond nearer to electron withdrawing acetyl group are oxidized.

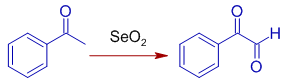
* Acetophenone can be oxidized with SeO2 to oxo(phenyl)acetaldehyde, a 1,2-dicarbonyl compound.