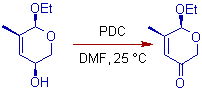* Pyridinium dichromate (PDC), (C5H5NH)2Cr2O7 is an orange colored solid used as an oxidizing agent.
* PDC is less acidic than PCC ( PyridiniumChloroChromate) and hence is more suitable in the oxidation of acid sensitive compounds.
* PDC in CH2Cl2 or DMF is also called as Corey-Schmidt reagent. In this case, PDC is maintained in anhydrous conditions.
* It is used for the selective oxidation of allylic alcohols in presence of other alcoholic groups. The oxidation product also depends on the solvent used.
In Dichloromethane, it conveniently oxidizes primary alcohols to aldehydes at room temperature.
Whereas, in DMF, a polar solvent, the non-conjugated primary alcohols are oxidized to carboxylic acids.
However conjugated alcohols like allylic and benzylic primary alcohols always give aldehydes only even in DMF.
Note: A mixture of CrO3, pyridine and water, which also forms PDC, is known as Cornforth reagent. This reagent contains water along with PDC. Hence it cannot be used in controlled oxidation of primary alcohols to aldehydes. However it can be used to oxidize secondary alcohols to ketones.
Structural Formula & Preparation
* Pyridinium dichromate, PDC can be obtained by gradual addition of a solution of chromic anhydride (CrO3) in water to pyridine in ice cold conditions.
However, the addition of anhydrous CrO3 to pyridine in ice cold conditions give dipyridine chromium(VI) oxide, CrO3.2C5H5N also called as Sarett's reagent.
Properties of reagent, Reaction conditions & Workup
Properties:
* Commercially, Pyridinium dichromate (PDC) is available as an orange solid. It is non hygroscopic. It is unaltered in open air.
* It is soluble in water, DMF and DMSO. But sparingly soluble in CH2Cl2 or CH3Cl.
Reaction condtions:
* The reaction conditions are mild and neutral. It can be employed to oxidize compounds containing acid sensitive functional groups.
* Even though the pyridinium cations are slightly acidic, very acid sensitive functionalities can withstand the action of PDC. However, some sodium acetate can be added as a buffer for a completely acid-free oxidations.
* The reactions are usually slow and some accelerators are needed. The activated molecular sieves; organic acids like acetic acid, pyridinium trifluoroacetate (PTFA), pyridinium tosylate (PPTS) etc.; acetic anhydride are added to speed up PDC oxidations.
* Anhydrous conditions must be maintained strictly to avoid the formation of carboxylic acids. The solvent CH2Cl2, can be dried over PDC and may be stored over molecular sieves to get best results.
Workup:
* The final workup involves addition of diethyl ether and the precipitate (inorganic part) is decanted and washed with ether. The collected organic phases are filtered through a pad of Celite or Silica or Fluorisil. Then the organic phase is concentrated to get the final product, which may be further purified by chromatographic techniques.
Alternately, Diethyl ether is added to the reaction mixture and washed with aqueous solutions of NaHCO3, HCl or NaCl. Then the ether phase is dried over MgSO4 or NaSO4 and concentrated to get the final residue which may need further chromatographic purification.
* Chromium (VI) compounds are carcinogenic and hence must be handled with care.
MECHANISM
* For the details of mechanism refer to jones reagent.
APPLICATIONS
* Primary alcohols are conveniently oxidized to aldehydes with Pyridinium dichromate, PDC in dichloromethane at room temperature.
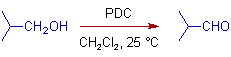
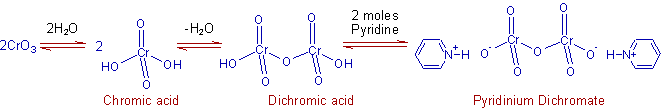
* Acid sensitive groups, such as enol ethers, are not affected during the oxidation with PDC.
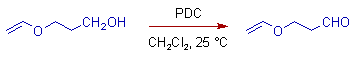
* Non-conjugated primary alcohols are oxidized to carboxylic acids with excess of PDC in moist DMF. But in Dichloromethane, the oxidation stops at aldehyde level only.
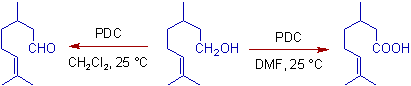
* However the conjugated primary alcohols are only oxidized to aldehydes even in DMF.
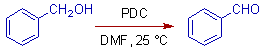
* The secondary alcohols are oxidized to ketones with Pyridinium dichromate, PDC.