* Lithium aluminium hydride, LiAlH4, also abbreviated as LAH, is a reducing agent commonly employed in modern organic synthesis.
* It is a nucleophilic reducing agent, best used to reduce polar multiple bonds like C=O.
* LiAlH4 reagent can reduce aldehydes to primary alcohols, ketones to secondary alcohols, carboxylic acids and esters to primary alcohols, amides and nitriles to amines, epoxides to alcohols and lactones to diols.
* Lithium aluminium hydride, LAH reagent cannot reduce an isolated non-polar multiple bond like C=C. However, the double or triple bonds in conjugation with the polar multiple bonds can be reduced.
* LiAlH4 is a powerful reducing agent compared to sodium borohydride, NaBH4, since the Al-H bond is weaker and thus less stable than B-H bond.
1) Structure of Lithium aluminium hydride
4) Mechanism of Lithium aluminium hydride - LiAlH4 reduction
5) Applications of LiAlH4 in organic synthesis
Structure of Lithium aluminium hydride - LiAlH4
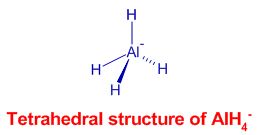
There is a tetrahedral arrangement of hydrogens around Al3+ in aluminium hydride, AlH4- ion. It is formed by coordination of hydride, H- ions to Al3+ ion. The hybridization in central Al is sp3.
Preparation of LiAlH4
LiAlH4 is prepared by the reaction between lithium hydride and aluminium chloride.

Properties of LiAlH4 , Reaction conditions & Workup
* Lithium aluminium hydride, LAH is a white solid but the commercial samples are usually gray due to presence of impurities.
* It reacts violently with water by producing hydrogen gas. Hence it should not be exposed to moisture and the reactions are performed in inert and dry atmosphere.

* The reduction reaction employing LiAlH4 as reducing agent must be carried out in anhydrous non protic solvents like diethyl ether, THF etc. It is highly soluble in diethyl ether. However it may spontaneously decompose in it due to presence of catalytic impurities. Therefore the preferred solvent for LAH is THF despite the low solubility.
* The reactions are usually performed with excess of LiAlH4. A small amount of the reagent is added to the solvent to eliminate any moisture present in the solvent.
Workup:
During the workup, the reaction mixture is initially chilled in an ice bath and then the Lithium aluminium hydride is quenched by careful and very slow addition of ethyl acetate followed by the addition of methanol and then cold water.
Sometimes, the reagent is decomposed by adding undried solvent slowly and then dilute sulphuric acid to the reaction mixture.
MECHANISM OF REDUCTION BY LITHIUM ALUMINIUM HYDRIDE, LiAlH4
* The reduction of a carbonyl group by LiAlH4 is initiated by the attack of nucleophilic hydride ion on the carbonyl carbon to give a tetrahedral intermediate.
* LiAlH4 is a nucleophilic reducing agent since the hydride transfer to the carbonyl carbon occurs prior to the coordination to the carbonyl oxygen. It reacts faster with electron deficient carbonyl groups. The reactivity of carbonyl compounds with this reagent follows the order:
Aldehydes > Ketones > ester > amide > carboxylic acid
* The steps involved in the reduction of various functional groups are shown below:
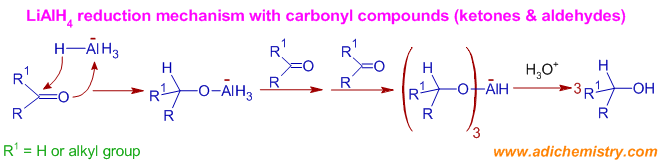
Mechanism of Reduction of Aldehydes or Ketones to 10 or 20 alcohols: Initially, a hydride ion is transferred onto the carbonyl carbon and the oxygen atom coordinates to the remaining aluminium hydride species to furnish an alkoxytrihydroaluminate ion, which can reduce the next carbonyl molecule. Thus three of the hydride ions are used up in reduction.
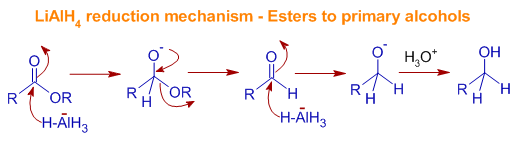
Mechanism of Reduction of Esters to 10 alcohols by LiAlH4: The ester is first converted to aldehyde which is further reduced to primary alcohol.
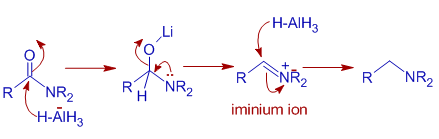
Mechanism of Reduction of Amides to amines: Amides are converted to amines. The LAH reduction mechanism is slightly different from that depicted for esters. In iminium ion is formed during the reaction since nitrogen atom is relatively a good donor than oxygen atom.
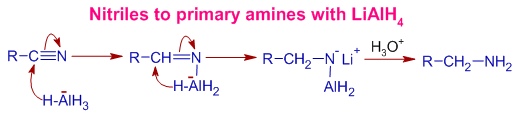
Mechanism of Reduction of nitriles to primary amines by LiAlH4: Initially, the polar CN bond is added with LAH such that the negatively charged hydride makes bond with carbon. It is followed by subsequent transfer of hydride from AlH3- group. Final protic workup generates amine group.
APPLICATIONS OF LiAlH4 IN ORGANIC SYNTHESIS
The summary chart of applications of LiAlH4 in the reduction of different types of functional groups.
| Functional group conversion | equivalents of LiAlH4 |
| Aldehydes, ketones -------> Alcohols | 1 |
| Carboxylic acids -------> Alcohols | 3 |
| Esters, acid halides -------> Alcohols | 2 |
| Amides -------> amines | 2 |
| Nitriles -------> amines | 2 |
| oxiranes (epoxides) -------> alcohols | 1 |
| lactones -------> diols | 2 |
| haloalkanes, haloarenes -------> alkanes, arenes | 1 |
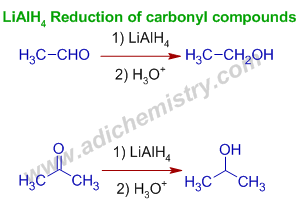
1) Reduction of carbonyl compounds using LiAlH4: The aldehydes or ketones are reduced by LiAlH4 to the corresponding primary or secondary alcohols respectively.
E.g. Acetaldehyde is reduced to ethyl alcohol and acetone is reduced to isopropyl alcohol.
* LiAlH4 does not affect the isolated carbon-carbon double or triple bonds.
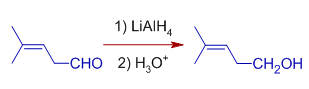
*However, the double bonds in conjugation at α,β positions of carbonyl group may also be reduced by Lithium aluminium hydride depending on the reaction conditions.
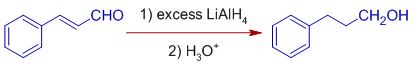
E.g. Cinnamaldehyde is reduced to Hydrocinnamyl alcohol when reduced with excess of LiAlH4 (roughly more than 2 equivalents) by normal addition method. In this method, a solution of cinnamaldehyde is added to the solution of lithium aluminium hydride. Both the double bond and carbonyl group are reduced.
Whereas, Cinnamaldehyde is reduced to Cinnamyl alcohol with one equivalent of LiAlH4 in inverse addition method. In this method, the solution of LiAlH4 is added to the solution of Cinnamaldehyde. Only the carbonly group is reduced to alcohol.
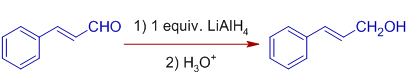
Stereochemistry:
The axial attack of hydride ion is preferred over the equatorial attack in case of cyclic systems. For example, 4-t-butylcyclohexanone yields more than 90% of trans-4-t-butylcyclohexanol when reduced with Lithium aluminium hydride.
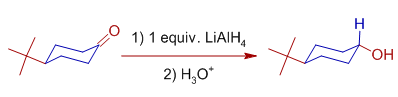
The plausible explanation for this behavior is: the -OH group prefers the equatorial position to avoid the interactions with other axial hydrogens. i.e., It is not the approach of hydride ion but the orientation of -OH group which decides the final stereochemistry.
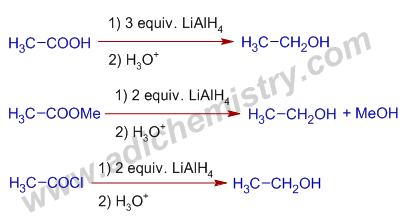
2) The carboxylic acids, esters and acid halides are reduced to corresponding primary alcohols by Lithium aluminium hydride.
E.g. The reduction of Acetic acid, methyl acetate and acetyl chloride by LiAlH4 furnish the same ethyl alcohol.
3) The amides are reduced to amines by Lithium aluminium hydride, LiAlH4. Especially this method is used to get secondary amines.
E.g. Diethyl amine can be prepared starting from acetyl chloride as follows:
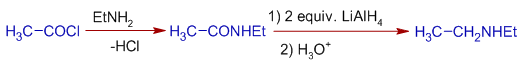
4) The nitriles are reduced to primary amines by LiAlH4.
E.g. Acetonitrile is reduced to ethyl amine by LiAlH4.
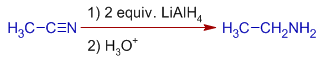
5) Lithium aluminium hydride reduces the oxiranes (epoxides) to alcohols. The mechanism involves hydride attack occurs at less hindered side of the epoxide.
E.g. 2-methyloxirane gives 2-propanol predominantly.
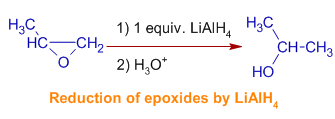
In case of cyclohexene epoxides, the axial alcohols are formed preferentially.
E.g.
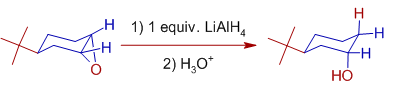
6) The lactones are reduced to α,ω-diols by LiAlH4.
E.g.
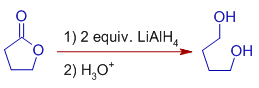
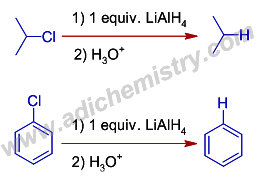
7) The haloalkanes and haloarenes are reduced to corresponding hydrocarbons by Lithium aluminium hydride.
TEST YOUR UNDERSTANDING - Lithium Aluminium Hydride Reagent
1) What happens to the pH of water when LiAlH4 is is added to it?
Answer:
The H-, hydride ions can react violently with water to liberate hydrogen gas and the solution becomes alkaline, now containing LiOH and Al(OH)3. Thus pH is increased. It will be greater than 7. The reaction of Lithium aluminium hydride, LiAlH4 with water is shown below.
LiAlH4 + 4H2O -------> LiOH + Al(OH)3 + 4H2
It is strongly basic and hence can not only react with water but also with protic solvents like methanol.
2) Why do we quench the reaction mixture with methanol after reduction with Lithium aluminium hydride?
Answer:
Just think.......what does LiAlH4 do with protic solvents? It can react with methanol in the same way as it reacts with water but less violently since the O-H bond in methanol is less polar.
LiAlH4 + 4MeOH -------> LiOMe + Al(OMe)3 + 4H2
Hence methyl alcohol is used in the quenching of LAH during workup. It is better to quench in cold conditions.
LITHIUM ALUMINIUM HYDRIDE - LiAlH4 REDUCTION- MCQ - IIT JEE - NEET - JAM - GATE - CSIR NET & SET EXAMS
1) The most appropriate reagent to convert RCOOEt -----> RCH2OH is: (GATE 1997)
A) LiAlH4
B) NaBH4
C) H2/Pd–C
D) Li/NH3(liq)
2) ethyl ethanoate on reduction with LiAlH4 gives
A) Ethanol
B) Diethyl ether
C) Ethane
D) Acetic acid and ethyl alcohol
3) When acetyl chloride is reduced with LiAlH4, the product formed is :
A) Ethanoic acid
B) Ethyl chloride
C) Ethyl alcohol
D) Ethane
4) The reduction product of N-ethylpropanamide with LiAlH4 is :
A) Diethylamine
B) 1-propanamine
C) N-ethylpropan-1-amine
D) N-ethylpropan-1-amine
5) The product formed when cyclopentanecarbaldehyde is reduced with LAH is :
A) cyclopentane
B) methylcyclopentane
C) ethylcyclopentane
D) cyclopentylmethanol
6) Reduction of 1-methylpyrrolidin-2-one with two equivalents of lithium aluminium hydride will give :
A) 1-methylpyrrolidine
B) 1-methylpyrrolidin-2-ol
C) pyrrolidin-2-ol
D) pyrrolidin-2-one
7) The reagent that can be used when 4-methoxybenzoic acid is reduced with LAH is :
A) Diethyl ether
B) Ethyl alcohol
C) THF
D) Water
E) Both A & C can be used
KEY & EXPLANATION
1) A
2) A
3) C
4) C
5) D
6) A
7) E