Silver carbonate, Ag2CO3 precipitated on Celite is called Fetizon's reagent. It is used to oxidize primary and secondary alcohols to aldehydes and ketones respectively under mild and neutral conditions on small scale. The Ag+ ion is reduced to metallic Ag0.
PREPARATION OF FETIZON'S REAGENT
The Fetizon's reagent is prepared as follows:
i) Initially Celite is purified by washing with methanol containing HCl and then with distilled water.
Note: Celite is the diatomaceous or siliceous earth mainly composed of silica.
ii) Purified Celite is added to silver nitrate solution in distilled water and then a solution of sodium carbonate is added to it slowly with stirring. The yellow green precipitate obtained is filtered off and dried.
The precipitate containing silver carbonate over celite is used as Fetizon's reagent. It has enhanced reactivity and can be filtered easily.
REACTION CONDITIONS & WORKUP
The reaction with Fetizon's reagent is carried out just by refluxing the reaction mixture in neutral apolar solvents such as benzene or heptane and the product is recovered in high purity by simply filtering off the Ag0 formed and evaporating the solvent.
The use of polar solvents with weak basic nature like ethyl acetate, diethyl ether etc., must be avoided since they may inhibit the oxidation by complexing to the silver ions and prevent the initial chemisorption of alcohol over surface of the reagent.
The water produced during the oxidation must be removed by performing azeotropic distillation using Dean-Stark apparatus. Otherwise it may inhibit the action of Fetizon's reagent by competing with the alcohol for complexing with silver ions.
Usually excess of the reagent is employed during the oxidation reaction.
REACTION MECHANISM
The oxidation with Fetizon's reagent is believed to occur through chemisorption of alcohol over the surface of silver carbonate. The oxygen atom of the -OH group and the α-hydrogen atom complex to the Ag+ ions, which are subsequently reduced to Ag0.
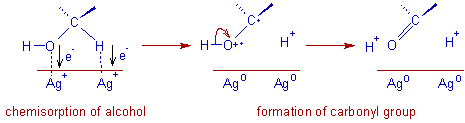
The overall reaction can be represented as:
![]()
APPLICATIONS OF FETIZON'S REAGENT
The Fetizon's reagent is used to oxidize alcoholic groups selectively in presence of non-polar functional groups. However the polar functional groups, may retard the rate of the reaction though not affected by the reagent. This is because of their complexing ability with the silver ions.
* The ease of oxidation of alcohols by Fetizon's reagent follows the trend: allylic, benzylic alcohols > 2' alcohols> 1' alcohols.
* However highly hindered -OH groups are not oxidized.
* The treatment of diols with Fetizon's reagent usually promotes oxidation of only one of the -OH group.
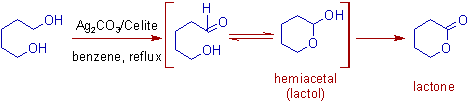
* The α,ω-diols (like1,4; 1,5; 1,6) containing two primary alcoholic groups give corresponding lactones via lactols. Initially, one of the hydroxyl group is oxidized to aldehyde, which cyclizes to an intermediate lactol. Further oxidation of lactol furnishes lactone.
ILLUSTRATIONS
1) The secondary hydroxyl groups react faster than the primary hydroxyl groups with Fetizon's reagent. Hence it is used to selectively oxidize secondary alcoholic groups as illustrated below.
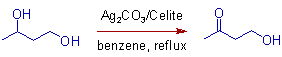
2) The allylic alcoholic groups are selectively oxidized in presence of primary and secondary alcohols.
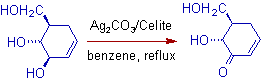
3) The treatment of 1,5-pentanediol with Fetizon's reagent furnishes a δ-lactone. Initially one of the -OH group is oxidized to yield a hydroxyaldehyde that equilibrates with a small amount of hemiacetal, which is further oxidized to an δ-lactone.