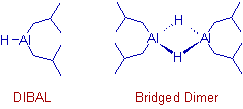
* Diisobutylaluminium hydride, iBu2AlH also known as DIBAL or DIBAL-H or DIBAH is an exceedingly useful and versatile reducing agent.
* It is an electrophilic reducing agent, usually employed in selective reductions of esters or nitriles to aldehydes; lactones to lactols; α,β-unsaturated carbonyl compounds to allylic alcohols, at low temperatures (-78oC).
* DIBAL-H reduces carbonyl or nitrile groups selectively in presence of double bonds, halide groups, ethers, nitro groups etc.,
DIBAL is an organoaluminium hydride existing as a dimer, (iBu2AlH)2 or as a trimer, (iBu2AlH)3.

DIBAH can be prepared by heating Triisobutylaluminium, TIBAL. It is a β-hydride elimination reaction.
![]()
* Commercially, DIBAL can be obtained as a neat colorless liquid or as a solution in hydrocarbon solvents like toluene.
* It is miscible with numerous solvents. Diethyl ether, THF, methylene chloride, chlorobenzene, toluene,hexane etc., are the suitable solvents.
* But it undergoes rapid oxidation in air and reacts vigorously with hydroxylic compounds such as water, alcohol etc. Hence the reductions with DIBAL should be carried out in the absence of air and moisture.
* The workup involves slow quenching with methanol followed by complete quenching with water. Alternatively, dil.HCl can be employed. Methanol destroys excess of DIBAL-H.
* DIBAL is said to be an electrophilic reducing agent because of its coordination to the carbonyl oxygen prior to the transfer of hydride onto carbonyl carbon. Hence it reacts fast with electron rich carbonyl groups.
Note: In contrast, LiAlH4 is a nucleophilic reducing agent since the hydride transfer occurs prior to the coordination to the carbonyl oxygen. It reacts fast with electron deficient carbonyl groups.
* At low temperatures (-78oC), the reduction of esters, nitriles and lactones can be stopped after the transfer of one hydride to the carbonyl carbon. It is because the tetrahedral intermediate formed is stable at low temperatures. This tetrahedral intermediate will furnish the corresponding aldehyde only upon hydrolytic workup.
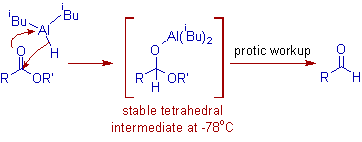
* But at higher temperatures, the carbonyl compound takes one more hydride and thus by forming alcohols finally. Hence DIBAL can also be used to reduce carbonyl compounds to alcohols.
Following is the summary chart of application of DIBAL-H in the conversion of different types of functional groups.
| Functional group conversion | Reaction conditions | |
| equivalents of DIBAL-H | Temperature | |
| Aldehydes, ketones -------> Alcohols | 1 | higher |
| Carboxylic acids -------> Alcohols | 3 | higher |
| Esters -------> Alcohols | 3 to 4 | higher |
| Carboxylic acids -------> Aldehydes | 2 | lower |
| Esters -------> Aldehydes | 1 | lower |
| Lactones -------> Lactols | 1 | lower |
| α,β-unsaturated esters -------> Allylic alcohols | 2 | lower |
| Nitriles -------> Aldehydes | 1 | lower |
| Nitriles -------> Amines | 2 | lower |
1) Aldehydes, Ketones, Carboxylic acids & Esters to Alcohols: At ordinary temperatures, DIBAL-H reduces variety of carbonyl compounds, like aldehydes, ketones, carboxylic acids and esters, to corresponding alcohols. These reductions are chemoselective as well as stereospecific.
One or less than one equivalent of DIBAL-H is required for the reductions of aldehydes or ketones.
E.g. i) Cinnamaldehyde can be reduced to cinnamyl alcohol. It is observed that only one-third of an equivalent of DIBAL-H is required and isobutylene is formed as a byproduct. This indicates, not only the hydrogen on aluminium but also the β-hydrogen on isobutyl groups participates in the reduction. But the double bond is intact during the reduction. A case of chemoselectivity.
Thus DIBAL-H is the reagent of choice for the reduction of α,β-unsaturated carbonyl compounds to allylic alcohols.

E.g. ii) Isoborneol is formed as major product when camphor is reduced with DIBAL-H. It is a stereospecific reaction. The hydride attack occurs from the less hindered side of the carbonyl group.

Carboxylic acids require 3 moles of DIBAL-H for their conversion into alcohols at higher temperatures. One equivalent is consumed for the formation of salt of carboxylic acid. The rest for the conversion into alcohol.
E.g. iii) Benzoic acid requires 3 equivalents of DIBAL-H for its conversion into benzyl alcohol.
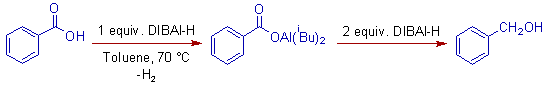
But in case of esters, 3 to 4 equivalents of DIBAL-H are required at ordinary temperatures. It may be due to formation of two alkoxyalumino intermediates, which then give corresponding alcohols.
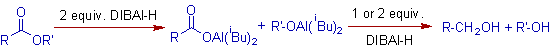
2) Carboxylic acids or Esters to Aldehydes: However, at lower temperatures (around -78oC), carboxylic acids (with 2 equiv. of DIBAL-H) or esters (with 1 equiv. of DIBAL-H) are selectively reduced to aldehydes (see the explanation above in mechanism section).
E.g. i) Benzoic acid is reduced to benzaldehyde with two equivalents of DIBAL-H at -70oC.
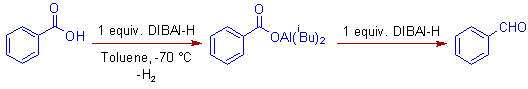
E.g. ii) Ethyl phenylacetate is reduced to phenyl acetaldehyde with only one equivalent of DIBAL-H at -70oC.

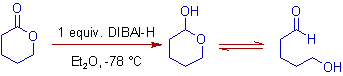
3) Lactones (cyclic esters) are reduced to lactols (cyclic hemiacetals) with DIBAL-H at low temperatures.
4) α,β-unsaturated carbonyl compounds are reduced to allylic alcohols with DIBAL-H.
E.g. Following α,β-unsaturated ester is converted to allylic alcohol with 2 equivalents of DIBAL-H at low temperatures (is not converted to aldehyde!?).
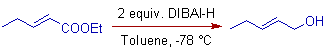
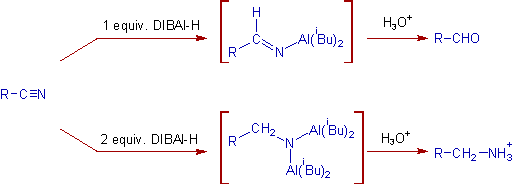
5) Nitriles to aldehydes or primary amines: The nitriles are either reduced to aldehydes or primary amines depending on the amount of DIBAL-H consumed.
Nitriles are reduced to aldehydes when treated with one equivalent of DIBAL-H at low temperature (-780C) followed by hydrolytic workup.
But they produce primary amines upon treatment with two equivalents of DIBAL-H, usually at room temperature, followed by protic workup.
E.g. i) In the following reduction, the double bond is intact while the nitrile is converted to aldehyde.
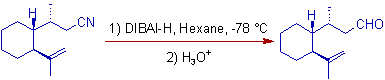
E.g. ii) 3,4-Dicyanofuran can be reduced to corresponding dialdehyde with two equivalents of DIBAL-H.
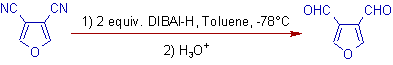
E.g. iii) Benzonitrile is reduced to Benzylamine when 2 equivalents of DIBAL-H are used at 110oC.
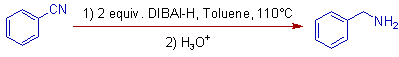
6) DIBAL-H can also be used in reduction of: alkynes to cis-alkenes; terminal olefins to alkanes.
Author: Aditya vardhan Vutturi