* The Jones reagent is a mixture of chromic anhydride and dilute sulfuric acid (CrO3 + H2SO4 + H2O) in acetone. It is used in the oxidation of secondary alcohols, that do not contain acid sensitive groups, to corresponding ketones.
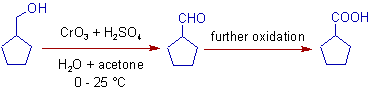
* The primary alcohols are initially oxidized to aldehydes, which are finally oxidized to carboxylic acids.
* A mixture of sodium dichromate or potassium dichromate in dilute sulfuric acid and acetone can also be used as Jones reagent.
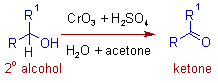
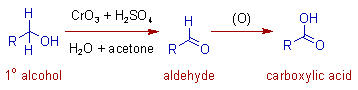
* This oxidation is usually referred to as Jones oxidation.
Reaction conditions & Workup
* The Jones reagent is prepared by adding chromic anhydride to dilute sulfuric acid in acetone and is added to the alcohol at 0-25oC.
* The orange or yellow colored Cr(VI) is reduced to blue green Cr(III) species during the oxidation.
* The excess Cr(VI), if any is remained, is destroyed in the reaction workup by adding isopropyl alcohol.
MECHANISM OF JONES REACTION
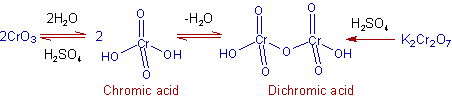
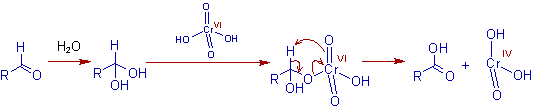
* Initially, chromic acid(VI) is generated is situ from the mixture of chromic trioxide and dilute sulfuric acid.
* The alcohol and chromic acid form chromium (VI) monoester, which may react intra-molecularly or inter-molecularly in presence of a base (H2O in this case) to give the corresponding carbonyl compound and chromium(IV) acid. The intra-molecular reaction occurs by way of a β-elimination through a cyclic transition state.
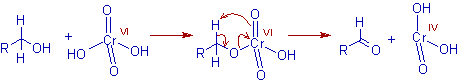
* The aldehydes, which can form hydrates in presence of water can further undergo oxidation to yield carboxylic acids in Jones reaction.
* Hence oxidation of primary alcohols with Jones reagent usually results in the formation of carboxylic acids due to presence of water. However benzyl and allyl alcohols do not for hydrates in water and hence can be selectively oxidized to aldehydes.
* If the oxidation is carried out in anhydrous conditions, it is possible to stop the reaction at aldehyde level. See at the end of the page for reagents which may serve this purpose.
* Follow up chemistry of Cr(IV) :The chromium(IV) acid is disproportionated into Cr(III) oxide and Cr(VI) acid.
![]()
APPLICATIONS OF JONES REAGENT
1) The secondary alcohols are oxidized to corresponding ketones in Jones reaction.
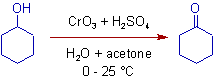
2) The primary alcohols are oxidized to carboxylic acids via aldehydes with Jones reagent.
3) Benzyl alcohol can be oxidized to benzaldehyde. Further oxidation to benzoic acid is not possible as the benzaldehyde cannot form stable hydrates in water.
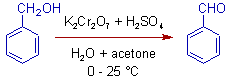
4) In Jones reaction, the allylic alcohols are also selectively oxidized to aldehydes. The double bonds are intact in this reaction.
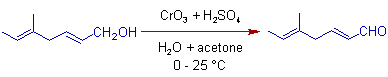
Some oxidizing reagents containing Cr(VI)
1) Sarett reagent: CrO3.2C5H5N (where C5H5N = pyridine)
2) Collins reagent: CrO3.2C5H5N diluted in CH2Cl2
3) Cornforth reagent: CrO3/ Pyridine / H2O
4) Fieser reagent: CrO3 in acetic acid.
5) Thiele reagent: CrO3 + acetic anhydride + H2SO4
6) Pyridinium Chlorochromate (PCC) in CH2Cl2 (Corey-Suggs reagent): [C5H5NH]+[CrO3Cl]-
7) Pyridinium Dichromate (PDC) in CH2Cl2 or DMF (Corey-Schmidt reagent): (C5H5NH)2Cr2O7