Answer:
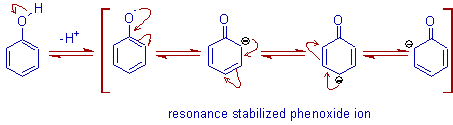
Phenols show acidic nature and hence are soluble in alkalies like NaOH, Na2CO3 etc. The acidic nature is due to formation of resonance stabilized phenoxide ion formed by losing a proton from -OH group.

When alkalies are added to phenol, the above equilibrium is shifted more to the right side as the H+ ions are removed by the OH- ions that are furnished by alkalies. Thus more and more phenol is converted to phenoxide ion that is soluble in water.
Water is a polar solvent. Hence polar substances like salts are more soluble in it. Phenoxide ion is more polar than phenol itself. Hence is more soluble in water.
Where from this water has come? We are adding alkalies and not water?
In general, when we are adding alkalies means we are adding their aqueous solutions and not the pure compounds.
What about the solubility of phenol in water? Is it soluble in it?
Yes. Phenol is also soluble in water to some extent. It is due to its ability to form hydrogen bonding with water molecules. However the large part of phenol molecule is phenyl group that is non polar and hence its solubility if limited in water.
However the polarity of this part too increases in phenoxide ion.
But phenol is not soluble in aqueous solution of NaHCO3. Why?
NaHCO3 is not strong alkali and hence it cannot provide as much OH- ions that are required to shift the above mentioned equilibrium. Hence only small amount of phenoxide is formed. Hence phenol is sparingly soluble in NaHCO3.
What are the other factors that affect the solubility of phenols in aqueous solutions?
Acidic strength. The solubility of phenols increase with increase in the acidic strength. It is enhanced when -I and -M groups (like -Cl, -Br, -NO2) are introduced on the ring particularly at ortho and para positions. These groups will reduce the negative charge on the phenoxide ion and thus stabilize it. They enhance the likeliness of formation of phenoxide ion.
1) What is resonance?
2) What are -I and -M groups?
3) Why polar substances are more soluble in polar solvents?
4) What will happen when phenol is mixed with aniline that is basic in nature?
Author: Aditya vardhan Vutturi