According to this model, an atom has a spherical shape in which the positive charge is uniformly distributed. The electrons are embedded into it in such a manner as to give the most stable electrostatic arrangement.
However this model could not explain the α-ray scattering experiment (described below).
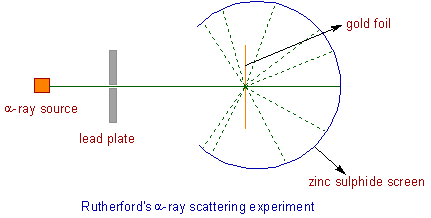
α- Ray scattering experiment:
A narrow beam of α- particles is passed through a thin gold foil which is surrounded by circular screen made up of fluorescent zinc sulphide. Whenever an α- particles strike the screen, a tiny flash of light will be produced at that point.

Observations:
1) Most of the α- particles pass through the foil without deflection.
2) A small fraction of the α- particles were deflected by small angles.
3) A very few α- particles bounced back i.e. were deflected almost by 180o.
Rutherford's atomic model:
1) Most of the space in the atom is empty.
2) The positive charge in the atom is concentrated in the small dense portion called the nucleus.
3) Electrons revolve around the nucleus in circular paths called orbits. It resembles the solar system.
4) Electrons and the nucleus are held together by electrostatic forces of attraction.
Drawbacks:
1) Rutherford’s model could not explain the stability of atom. According to electromagnetic theory, the charged particle under acceleration should continuously emit radiation. Hence the electron moving in the orbits must lose energy and fall into the nucleus. But this is not happening.
2) This model could not explain the electronic structure and energy of electrons.
3) It could not explain the atomic spectra.
The modern view of atomic structure is based on quantum theory. A brief discussion of nature of light and quantum theory is presented on the next page.
Author: Aditya vardhan Vutturi