While it is taught that the chemical bonds are divided broadly into ionic and covalent types, however, in reality, most of the bonds are neither purely ionic nor purely covalent. These terms are used to indicate two extreme cases. The ionic bond refers to the complete transfer of electrons from one atom to the other, whereas the pure covalent bond involves equal sharing of electrons. Nevertheless, the nature of chemical bonds in most of the compounds are somewhere in between above said two extremes.
In general, it is expected that the ionic compounds are soluble in polar solvents like water and show high melting and boiling points as well as exhibit electrical conductivity in the liquid state. As a rule, these compounds should be sparingly soluble in nonpolar solvents like benzene, carbon tetrachloride, etc. However, it is observed that few of the ionic compounds are also fairly soluble in them and also exhibit somewhat less than anticipated melting points and electrical conductivity in the molten state.
It is also observed that some of the covalent compounds are soluble in water and exhibit electrical conductivity, though not very much, in the molten state.
This clearly shows more or less covalent nature is also possible for the ionic compounds and vice versa. These observations can be explained by the concept of polarization of anion and thus led to the formulation of Fajan's rules (or Fazans rules) which help in deciding the nature of chemical bonds.
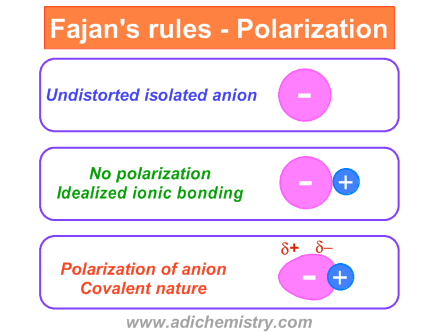
The negative charge on an isolated anion is evenly distributed around the nucleus. But in the presence of a cation, the electron density is distorted towards it. Thus the negative charge is unevenly distributed over the anion. One end of the anion gets a relatively more negative charge than the other end. This condition is referred to as polarization.

Note that the cation is also slightly polarized in the presence of anion. Due to polarization, the electron density is now more spread out in between the anion and cation. This condition is more or less similar to the covalent bonding.
Fajan's rules were developed based on the concept: "the greater the polarization of anion, the greater is the covalent nature". There are two factors which are crucial in deciding the extent of polarization as mentioned below.
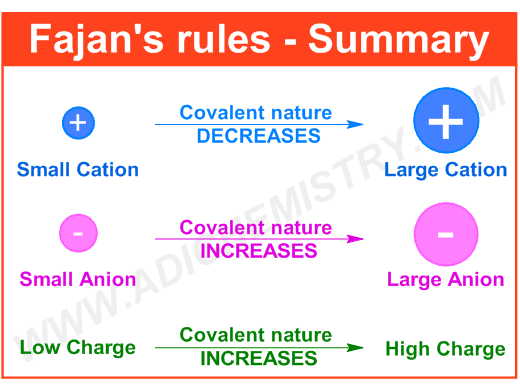
The ability of a cation to polarize the anion is referred to as polarizing power. It is directly proportional to the charge density, which in turn is directly related to the charge on cation, while inversely related to the size of anion.
The polarizing power increases with increase in the size of cation i.e. smaller cations are very effective in the polarization of anion.
However, the polarizing power increases with increase in the charge on cation.
It is the tendency of an anion to undergo polarization. It indicates the easiness with which an anion undergoes distortion in presence of a cation.
It is directly proportional to the size as well as the negative charge on the anion.
The larger anions can undergo distortion very easily than the smaller ones.
It is also important to note that the anions with greater negative charge also undergo polarization easily.
We can easily conclude that greater the polarizing power of cation and greater the polarizability of anion, greater is the polarization and hence greater will be the covalent nature. Based on above discussion now we can write Fajan's rules.
Due to polarization of anion and the increase in covalent nature, the ionic compounds exhibit abnormal behavior as mentioned below.
It is observed that the ionic compounds with covalent nature are appreciably soluble in the non-polar solvents with low dielectric constants.
The melting points of ionic compounds with covalent character are less than anticipated.
Due to polarization, the anions may undergo easy oxidation when exposed to certain conditions. The polyatomic oxyanions like carbonates, sulfates, etc. are distorted very easily in presence of cations with greater polarizing power and therefore are decomposed very easily upon heating.
We know that the size of cations increases from top to bottom in a given group of periodic table. Therefore the cation size of alkali metals also increases as shown below.
Li+ < Na+ < K+ < Rb+ < Cs+
Therefore the covalent nature of alkali metal halides containing the same halide ion decreases from Lithium halide to Cesium halide according to Fazan's rule-1.
For example, the decreasing order of covalent nature of alkali metal chlorides is:
LiCl > NaCl > KCl > RbCl > CsCl
Once should also note that the solubility of these compounds in non polar solvents with low dielectric constant also decreases in the same order.
While the melting points follow the reverse order due to the decrease in covalent character (or increase in ionic nature).
When the alkali metal ion is fixed while the halide is changed, the trend in covalent nature depends on the size of anion.
For example the order of covalent character for Lithium halides with different halide ions is as shown below.
LiF < LiCl < LiBr < LiI
In the above case, the size of anion increases from fluoride to iodide ion and hence the covalent nature increases.
The stability of these compounds, however, decreases from LiF to LiI due to increase in polarization of anion.
Indeed, Lithium iodide becomes yellow when exposed to air due to oxidation of Iodide to Iodine. It is due to strong polarization of larger Iodide anion in presence of smaller lithium ion.
The covalent nature of chlorides of Na, Mg & Al is in the following order.
NaCl < MgCl2 < AlCl3
It is due to increase in the positive charge on cations i.e. Na+ < Mg2+ < Al3+.
When the cations have octet configuration or inert gas configuration (ns2np6) in their outer shells, the effective nuclear charge properly shielded by the inner s and p electrons. Hence their polarizing power is less than expected. Therefore the ionic compound with these cations have less covalent character.
Whereas, the nuclear attraction is not properly shielded when there are electrons in the d-sub-level. Therefore the cations with pseudo-octet configuration i.e. ns2np6nd10 posses high polarizing power.
Therefore the main group metal compounds are more ionic while the transition metal compounds are more covalent.
For example, CaCl2 is more ionic due to the inert gas configuration (3s23p6) in the Ca2+ ion. While the ZnCl2 is more covalent due to pseudo-inert gas configuration in the Zn2+ ion (3s23p63d10).
1) According to Fajan's rules, the covalent nature of ionic compounds is favoured by [AIIMS 1999]
A) Large cation and small anion
B) Large cation and large anion
C) Small cation and large anion
D) Small cation and small anion
Answer: C
2) Even though the crystal radii of both K+ and Ag+ are almost same, the melting point of AgCl is only 455°C, while that of KCl is 776°C. The best explanation is:
A) AgCl has more ionic character than KCl due to greater polarizing power of potassium ion.
B) KCl has more ionic character than AgCl due to greater polarizing power of potassium ion.
C) AgCl has more covalent character than KCl due to greater polarizing power of silver ion.
D) KCl has more covalent character than AgCl due to greater polarizing power of silver ion.
Answer: C
Explanation: The K+ ion has octet configuration in the outer shell (3s23p6). Therefore it has less polarizing power and hence KCl is more ionic. Whereas, in Ag+ ion, there is pseudo octet configuration (4s24p64d10) in its outer shell. Therefore it has greater polarizing power due to ineffective shielding of nuclear charge by d-electrons.
3) The order of covalent nature for aluminium halides is AlF3 < AlCl3 < AlBr3 < AlI3. The best explanation according to Fajan's rules is:
A) Al3+ is larger in size and hence it has greater polarizing power.
B) The polarizability of halide ions increases with increase in their size and hence the covalent nature also increases.
C) The polarizability of halide ions decreases with increase in their size and hence the covalent nature decreases.
D) The given order of covalent nature is due to greater polarizing power of Al3+ ion.
Answer: B
4) The correct order of covalent nature of alkali metal chlorides is:
A) LiCl < NaCl < KCl < RbCl < CsCl
B) CsCl < NaCl < KCl < RbCl < LiCl
C) LiCl > NaCl > KCl > RbCl > CsCl
D) LiCl = NaCl = KCl = RbCl < CsCl
Answer: C
Explanation: The covalent nature decreases with increase in the size of cation since the polarizing power decreases with increase in the size.
5) Polarization is the distortion of the shape of an anion by an adjacently placed cation. Which of the following statements is correct: [NCERT 1982]
A) Maximum polarization is brought about by a cation of high charge
B) A large cation is likely to bring about a large degree of polarization
C) Minimum polarization is brought about by a cation of low radius
D) A small anion is likely to undergo a large degree of polarization
Answer: A - From Fajan's rules
6) Which of the following has a high polarising power? [CET Pune 1998]
A) Mg2+
B) Al3+
C) Na+
D) Ca2+
Answer: B
Explanation: According to Fajan's rules, the greater the charge the greater is the polarizing power of cation.
7) Maximum covalent character is associated with the compound: [RPMT 1999]
A) NaI
B) MgI2
C) AlCl3
D) AlI3
Answer: D
Explanation: Among cations, Al3+ has smaller size and greater charge hence it has greater polarizing power. Among anions, iodide (I-) is larger and hence can be polarized easily. Hence the combination of these two ions i.e. Al3+ and I- results in more covalent nature.
8) According to Fajan's rules, the covalent bond is favored by [AIIMS 1999]
A) Large cation and small anion
B) Large cation and large anion
C) Small cation and large anion
D) Small cation and small anion
Answer: C
9) Polarisibility of halide ions increases in the order: [DCE 1999]
A) F-,I-,Br-,Cl-
B) Cl-,Br-,I-,F-
C) I-,Br-,Cl-,F-
D) F-,Cl-,Br-,I-
Answer: D
10) Amongst LiCl, RbCl, BeCl2 and MgCl2 the compounds with the greatest and the least ionic character, respectively, are:
A) LiCl and RbCl
B) RbCl and BeCl2
C) RbCl and MgCl2
D) MgCl2 and BeCl2
Answer: B
11) Polarization power of cation increases when :
A) size decrease
B) size increases
C) Anion has greater polarizing power
D) covalent nature increases
Answer: A
11) Polarising power is directly proportional to :
A) size of cation
B) charge on cation
C) electronegativity of cation
D) size of anion
Answer: B