All the atoms, except those of inert gases, participate readily in the formation of chemical bonding. It is necessary to understand the formation and nature of chemical bonds to arrive at the structures of molecules; study the interactions between them; and to understand the chemical reactivity.
Let us start with a few fundamental questions:
What is a chemical bond?
In simple words, the attraction between two atoms or ions that holds them together is known as chemical bond .
How is it formed?
A chemical bond may be formed either by sharing of electrons or by transfer of electrons between atoms i.e., by reorganization of electrons between atoms.
This is the major difference between intermolecular forces of attractions (van der Waals forces) and chemical bonds. In case of van der Waals forces of attractions, there will be no reorganization of electrons occurring between two atoms.
Why a chemical bond is formed?
A chemical bond is formed by an atom to get more stability. Every atom tries to get more stability by lowering its potential energy. This can be achieved by making a bond.
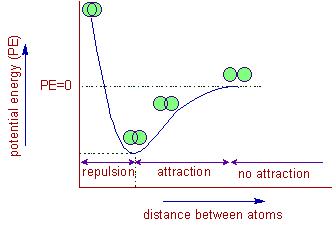
The potential energy decreases when two atoms attract each other. Hence energy is liberated during the formation of a chemical bond i.e., it is an exothermic process.

Note: Read the graph from right to left.
The potential energy of the atoms is arbitrarily fixed as zero when there is no attraction between them. As the two atoms start overlapping with each other, the potential energy also decreases and reaches a minima (move your eye from right to left). The distance between the nuclei of two atoms at this point is called bond length. The forces of attraction between two atoms are maximum at this point.
However the potential energy starts increasing again if the atoms are bring more closer than this distance, since repulsion forces start dominating the forces of attractions.
What is the connection between stability and electronic configuration?
The noble gas atoms with octet configuration in their outer shells are very stable. Hence every atom tries to get octet configuration either by losing or gaining or sharing electrons. This is also called as octet rule.
However this rule may not be followed always by the atoms.
The chemical bonds are broadly divided into:
3) Metallic bond
1) Ionic bond: The electrostatic force of attraction between two oppositely charged ions is called ionic bond.
* An ionic bond is formed due to transfer of electrons from one atom to another.
* The atom that loses electrons will form a cation and the atom that gains electrons will form an anion. These oppositely charged ions come closer to each other due to electrostatic force of attraction and thus by forming an ionic bond.
* An ionic bond is formed between two atoms when their electronegativity difference is greater than 1.7 on Pauling's scale.
* In general, an ionic bond is formed between a metal atom and a nonmetal atom.
E.g., NaCl, LiF, MgCl2 etc.,
2) Covalent Bond: The chemical bond formed between two atoms due to the sharing of electron pair(s) is called covalent bond.
* It is formed between two atoms for which the electronegativity difference is less than 1.7 on Pauling's scale.
* Usually two nonmetal atoms form a covalent bond.
E.g. H2, F2, HCl, H2O etc.
* The shared pair of electrons, also known as bond pair, is either formed due to equal contribution of electrons by each atom participating in the bond formation; or contributed by only one atom. In the later case, the bond is also known as co-ordinate covalent bond or dative bond.
3) Metallic bond: It is the attraction between metal atoms in a metallic crystal. It is formed between electropositive metal atoms of same or different elements. It is also considered as highly delocalized covalent bond.
E.g. The metal atoms Na, Cu, Ag, Fe etc. are bound to each other in their crystals by metallic bond.
Several theories were put forwarded to explain the chemical bonding. The important theories of chemical bonding are:
1) Electronic theory of chemical bonding: According to this theory proposed independently by Kossel & Lewis, a chemical bond between atoms is formed in order to get nearest inert gas configuration. This can be achieved by either losing or gaining or sharing electrons.
2) Valence bond theory: This theory of chemical bonding was first proposed by Heitler & London and developed by Linus Pauling. It explained the formation of covalent bonds between atoms, which is supposed to be formed due to overlapping of atomic orbitals.
3) Molecular orbital theory: This is rather an advanced treatment for chemical bonding. According to this theory, the atomic orbitals of bonding atoms combine with each other by giving rise to molecular orbitals.
Author: Aditya vardhan Vutturi