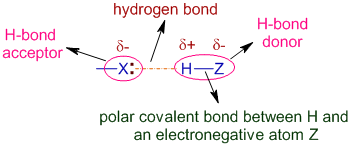
Definition: The electrostatic attraction between partially positively charged hydrogen atom bonded to a relatively electronegative atom and another electronegative atom is referred to as hydrogen bond.
It is represented by a dotted line as shown below.

* Z is an electronegative atom to which H atom is covalently bonded.
* The H-Z group is also called as donor group since it provides hydrogen atom for H-bonding.
* X is another electronegative atom that attracts positively charged H atom.
* X is referred to as hydrogen bond acceptor since it accepts positive charge from the H atom.
* The acceptor atom, X may or may not be bonded to another H atom covalently but should contain at least one lone pair on it.
Note: The donor and acceptor terminology can be a bit confusing since we are used to think about chemical bonds in terms of electrons. The H-bond donor provides H atom for making hydrogen bond but accepts the electron lone pair, whereas the H-bond acceptor receives H-atom and is actually the electron lone-pair donor.
* X and Z are smaller and highly electronegative atoms. They are usually, but not necessarily, from the first row of periodic table i.e. F, O and N.
The δ+ H atom attracts the lone pair electron density on X. This predominantly electrostatic force of attraction between δ+ H and Z is called as hydrogen bonding and is represented by a dotted line.
1) The hydrogen atom participating in H-bond must be covalently bonded to a highly electronegative atom in order to acquire significant positive charge.
For example, H atom covalently bonded to electronegative atoms like F, O and N can participate in hydrogen bonding.
However, H atoms bonded to less electronegative atoms like B, C etc. or larger electronegative atoms like S, P, Cl, Br etc. cannot get sufficient positive charge and hence cannot enter into H-bonding. Nevertheless there are some special cases.
2) The acceptor electronegative atom should also possess at least one lone pair and high charge density so as to attract positively charged H atom effectively. Thus H-bonding is normally observed with smaller and highly electronegative atoms such as F, O and N with lone pairs.
3) The H-bond is mostly electrostatic in nature. However, directional nature of hydrogen bonding, shorter inter atomic distances between molecules participating in H-bonding and limited number of H-bond interactions suggest some covalent nature also.
E.g. In HF, the number of H-bonds per each molecule is limited to only two, since there is only one hydrogen per molecule and only one lone pair on Fluorine can participate in H-bonding. The directional nature is also evident from the following diagram.
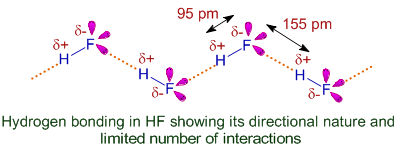
4) The hydrogen bond is stronger than van der Waal's forces of attraction. But it is weaker than the covalent bond and ionic bond.
The strength of Hydrogen bonds can range from very weak (1–2 kJ mol-1) to extremely strong (161.5 kJ mol-1). H-bonds involving fluorine are associated with higher energies. Most of the remaining types are below the range of 21-25 kJ mol-1.
5) The strength of H-bond is directly proportional to the electronegativity and the ability of electronegative acceptor atom to contribute the electron density.
| H-bond | Energy in vapor state |
| F-H----F | 161.5 kJ mol-1 |
| O-H----O | 21 kJ mol-1 |
| N-H----N | 13 kJ mol-1 |
| O-H----N | 29 kJ mol-1 |
| N-H----O | 8 kJ mol-1 |
The H-bond formed between two different molecules (of same or different compounds) is referred to as intermolecular H-bond. In this case, hydrogen of one molecule makes H-bond with electronegative atom of another molecule. These molecules may belong to same compound or different compounds.
E.g.
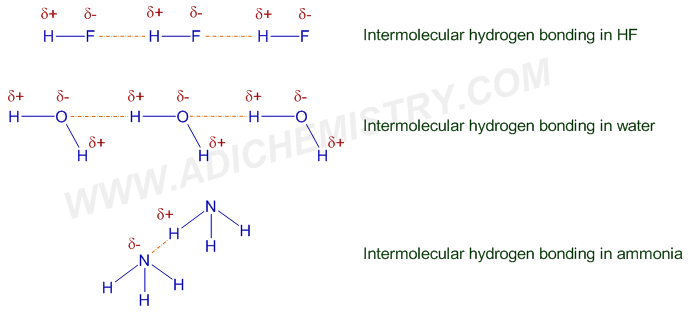
Note: In above diagrams, directional nature of H-bonding is not taken into account.
Consequences of intermolecular hydrogen bonding
1) The boiling point, melting point, sublimation point as well as enthalpy of vaporization of compounds increase due to intermolecular H-bonding.
E.g. The boiling point of H2O is high (373 K) due to intermolecular hydrogen bonding and hence it exist as liquid at room temperature, whereas H-bonds are absent in H2S and hence it has low boiling point and exists as a gas at room temperature.
Note: Though sulfur is an electronegative element, it cannot form hydrogen bonds due to larger size. There is no ample charge density on either sulfur or hydrogen to make a H-bond.
2) The solubility of compounds, showing intermolecular H-bonding, in water increases due to formation of intermolecular H-bonds with water.
E.g. Ethyl alcohol, methyl alcohol, ammonia, HF, acetic acid etc., are fairly soluble in water due to their ability to form intermolecular hydrogen bonds with water molecules.
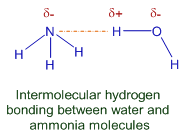
Intermolecular H-bonding between water and ammonia molecules is shown below.
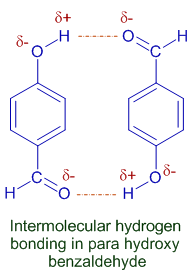
3) Due to intermolecular H-bonding, para isomers have higher boiling points and are fairly soluble in water. Therefore they cannot be separated by steam distillation.
E.g. Para hydroxy benzaldehyde has high boiling point and is more soluble in water, than its ortho isomer, due to intermolecular H-bonding and hence cannot be separated by steam distillation.
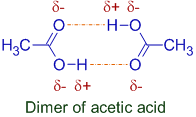
4) Some molecules exist as dimers or trimers or polymers in non-aqueous medium or in vapor state due to strong intermolecular H-bonding.
E.g. Acetic acid exists as dimer in benzene.
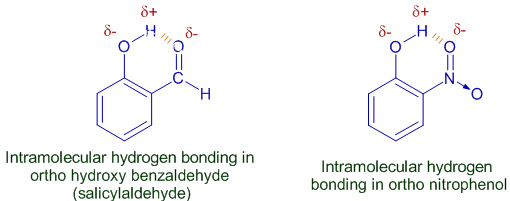
The H-bond formed between a hydrogen and the electronegative atom of same molecule is called as intra-molecular H-bond. This is also termed as intra-molecular chelation, since a ring is formed within the molecule.
E.g. Ortho hydroxy benzaldehyde and ortho nitro phenol show intra-molecular hydrogen bonding.
Consequences of intra-molecular hydrogen bonding
1) The boiling point, melting point and solubilities of isomers containing intra-molecular hydrogen bonds are lower than their isomers showing intermolecular H- bonds.
E.g. The boiling point and solubility of ortho hydroxy benzaldehyde (salicylaldehyde) are lower than those of para hydroxy benzaldehyde.
In ortho hydroxy benzaldehyde, there are intra-molecular hydrogen bonds, whereas para hydroxy benzaldehyde forms inter molecular H-bonds. Hence ortho hydroxy benzaldehyde is steam volatile and can be separated easily from other isomers by steam distillation.
In general, boiling point, melting point and solubility in water are lower for ortho isomers due to intra-molecular hydrogen bonding when compared to their para isomers.
Each water molecule can form four H-bonds in tetrahedral geometry due to presence of two lone pairs on oxygen and two hydrogen atoms as illustrated below.
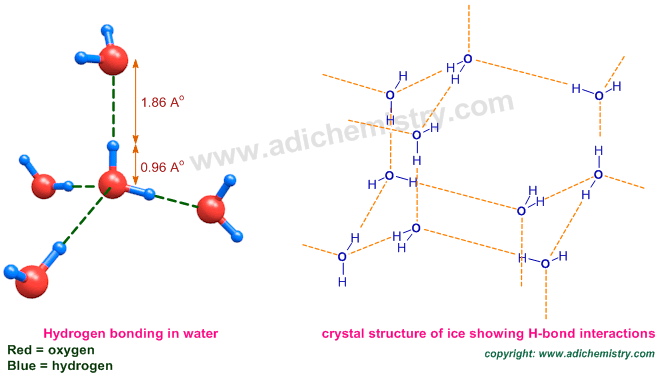
Why density of ice is less than water?
Solid water (ice) has less density than water due to stable hydrogen bonding which forces the crystalline structure to be very open (hexagonal type of) creating some extra interstitial space. Hence ice occupies more volume and hence less density than water.
Note: Water contracts until the temperature is decreased to 4 oC like any other liquid. Then it starts expanding with further decrease in temperature. It expands about 9% at its freezing point.
Remember that the hydrogen bonds in water are not stable. They constantly breakup and reform. But in ice they are stable and cannot be shaken out of position due to insufficient thermal energy available at low temperatures.
The boiling points of compounds increase with increase in the molecular weight since the van der Waal's forces of attraction also increases with increase in the size of molecule.
Hence we expect smooth increase in the boiling points of hydrides down the group. However, contrary to the expectation, the BP's of hydrides of group 15, 16 and 17 of the periodic table, are decreased from the first member to the next and then show regular increment while going down in each group.
The trend in BP's of hydrides of group 15 is:
NH3 > PH3 < AsH3 < SbH3 < BiH3
The order of BP's of hydrides of group 16 is:
H2O > H2S < H2Se < H2Te < H2Po
And the trend in BP's of hydrides of group 17 is:
HF > HCl < HBr < HI
The unexpected higher boiling points of NH3, H2O and HF are due to intermolecular hydrogen bonding. However the second hydride in each group i.e. PH3, H2S & HCl have low boiling points since they cannot form H-bonds due to larger size of central atoms which cannot get significant charge density to interact with a δ+ hydrogen atom even though they are also electronegative elements.
Alcohols, R-OH can form intermolecular hydrogen bonds due to presence of -OH group that acts as donor group for H-bonding, whereas ethers, R-O-R lack such groups and cannot exhibit H-bonding. Hence alcohols posses high boiling points and are fairly soluble in water. However, ethers show lower boiling points than their isomeric alcohols and are sparingly soluble in water.
H2O has more boiling point than HF even though the H-bonds in HF are more stronger and molar mass of HF (20.01 g mol-1) is greater than that of H2O (18 g mol-1).
Reasons:
1) In H2O, there are twice the number of hydrogen bonds per molecule than in HF.
2) HF exists as (HF)6 cluster of molecules even in vapor state. Hence it require less energy for transition from liquid state to vapor state. Less number of bonds have to be broken while liquid HF is converted to gaseous HF.
Hence the boiling point of H2O is higher than HF.
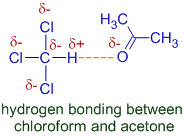
A mixture of chloroform and acetone shows negative deviation from Raoult's law. The vapor pressure of this mixture is less than expected due to intermolecular hydrogen bonding between these molecules.
Due to three electron withdrawing groups on carbon, which makes hydrogen more positive, hydrogen bonding between chloroform and acetone are now possible. This is a special case in which the hydrogen bonded to a carbon atom acts as H-bond donor.
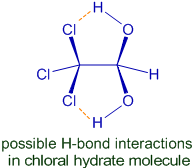
In general, the compounds containing two or more hydroxy groups (-OH) are unstable and tend to get stability by losing water molecule immediately. Hence the hydrates formed by addition of water molecule to carbonyl compounds are very less stable and form the original carbonyl group by losing a water molecule immediately.
However, chloral hydrate is reported to be a stable compound. It might be due to hydrogen bonding that exists between chlorine atoms and the hydrogens on hydroxy groups.
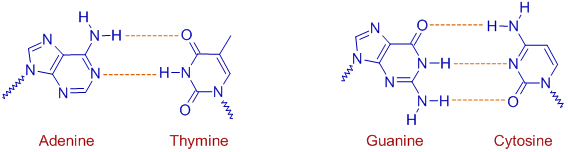
H-bonds plays major role in the formation of double helical structure of DNA. The hydrogen bonding between base pairs are responsible for the double stranded structure and those between phosphates cause the DNA strand to twist.
In DNA, it is observed that Adenine (A) always pairs with Thymine (T) and Guanine (G) always pairs with Cytosine (C) and hence the number of Adenine molecules is always equal to the number of Thymine molecules and the number of Guanine molecules is same as that of Cytosine molecules in DNA.
It is because, A forms two H-bonds with T, whereas G forms three hydrogen bonds with C. Other combinations will result in less number of H-bonds.
The hydrogen bonds also play major role in the secondary and tertiary structures of proteins. The carboxylic group oxygens and amide group hydrogens participate in the H-bonding and confer stable and specific structural conformations to the proteins.
Many carbohydrates like mono and disaccharides are soluble in water due to formation of intermolecular H-bonds with water. For example, glucose, fructose, sucrose, maltose, lactose etc. are fairly soluble in water.
Some polysaccharides also show strong affinity with water and form lyophilic colloids. E.g. Starch
The fibrous nature of cellulose can attributed to the formation of H-bonds.
Synthetic polymers like nylon are also strengthened by the hydrogen bonds.
Author: Aditya vardhan Vutturi