You might have seen the effervescence when lime juice is dropped on the floor, leaving a white mark. Or you might have observed the use of baking soda as a leavening agent to rise cookies, cakes etc.,. You may wonder about the fizz when club soda or coke bottles are opened. It is a known fact that our favorite bakery items are rendered tasty by adding baking powder. The use of washing soda in laundries, in softening hard water; and the use of lime stone and lime water in making construction materials like Portland cement, lime mortar etc., - all of these involve carbonates or bicarbonates.
Carbonates and bicarbonates find their way into household things to metallurgical processes and even biological reactions. They are present in tooth pastes, black board chalks, minerals, medicines etc.
- Formulae & structures
- Compounds containing carbonates & bicarbonates
- Occurrence
- Preparation
- General properties
- Chemical reactions
- Study of some individual compounds
FORMULAE & STRUCTURES OF CARBONATES AND BICARBONATE ANIONS
So after all, what are carbonates & bicarbonates?
Very simple. These are the salts of carbonic acid.
- Formula of Carbonate ion: CO32-
- Formula of Bicarbonate ion: HCO3- (It is also referred to as or hydrogencarbonate ion in IUPAC system)
These anions are formed from carbonic acid, H2CO3 by removing H+ ions successively as follows:
H2CO3 <-------> HCO3- + H+ <--------> CO32- + H+
The structural relationships can be represented as:
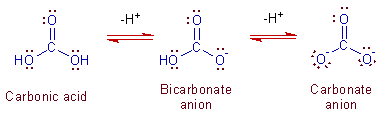
Their shapes are trigonal planar with 120o of bond angles at carbon atom. The central carbon atom undergoes sp2 hybridization.
COMPOUNDS CONTAINING CARBONATE OR BICARBONATE ANIONS
Usually metal ions with bigger atomic size form stable carbonates and bicarbonates. Some of the bicarbonates can only be detected in aqueous medium. Some important carbonates and bicarbonates are listed below.
| Group | compound | General formula | Examples |
| Group-1 (alkali metals) | carbonates | M2CO3 | Li2CO3, Na2CO3, K2CO3 etc., |
| bicarbonates | MHCO3 | LiHCO3, NaHCO3, KHCO3 etc., | |
| Group-2 (alkaline earth metals) | carbonates | MCO3 | MgCO3, CaCO3, BaCO3 etc., |
| bicarbonates | M(HCO3)2 | Mg(HCO3)2, Ca(HCO3)2 etc., | |
| p-block elements | carbonates | _ | Tl2CO3 and PbCO3 |
| Transition elements | carbonates | _ | ZnCO3, CuCO3, Ag2CO3, FeCO3 etc.,. |
There are several carbonate minerals present in the nature. A few of them are listed below.
| Formula | Name of the mineral |
| Na2CO3 | Soda ash or Natrite |
| CaCO3 | Lime stone or Calcite or Aragonite or Chalk |
| MgCO3 | Magnesite |
| CaCO3.MgCO3 | Dolomite |
| SrCO3 | Strontianite |
| BaCO3 | Witherite |
| PbCO3 | Cerrusite |
| FeCO3 | Siderite |
| CuCO3.Cu(OH)2 | Malachite |
| 2[CuCO3].Cu(OH)2 | Azurite |
| ZnCO3 | Smithsonite (in the old literature it is known as calamine) |
| CdCO3 | Otavite |
PREPARATION OF CARBONATES & BICARBONATES
Carbonic acid is formed when carbon dioxide gas is dissolved in water.
H2O + CO2 <--------> H2CO3
Though looking simple, this reaction is the basic principle involved in the manufacture of club soda, coca cola, Pepsi etc.,. These beverages are made by dissolving carbon dioxide gas in water at high pressures. Of course, some other ingredients are also added to improve the taste of the product. That is another story. When you open the bottle, the carbon dioxide gas will come out with effervescence (you call it fizz).
It is possible to get either carbonate or bicarbonate by passing carbon dioxide into alkaline solutions. Usually carbonates are formed when small amounts of carbon dioxide are passed through alkaline solutions.
E.g.
| 2NaOH | + | CO2 | <------> | Na2CO3 | + | H2O |
| small amount | fairly soluble in water |
| Ca(OH)2 | + | CO2 | <------> | CaCO3↓ | + | H2O |
| small amount | insoluble in water |
But bicarbonates are eventually formed when excess of carbon dioxide is passed into the solution.
E.g.
| NaOH | + | CO2 | <------> | NaHCO3 | ||
| excess | sparingly soluble
in cold water |
| Ca(OH)2 | + | 2CO2 | <------> | Ca(HCO3)2 | ||
| excess | soluble in water |
Application-1: It is observed that lime water, Ca(OH)2 turns milky initially when carbon dioxide is passed through it and becomes clear after passing excess of carbon dioxide. Initially an insoluble white solid, CaCO3 is formed. Hence lime water turns milky. It is then converted to water soluble bicarbonate, Ca(HCO3)2 upon passing excess of carbon dioxide by making the solution clear again.
The reactions are summarized below.
| Ca(OH)2 | + | CO2 | ----------> | CaCO3↓ | + | H2O |
| Slaked lime | small amount | white solid |
| CaCO3 | + | H2O | + | CO2 | ----------> | Ca(HCO3)2 |
| excess | soluble |
Note: The formation of calcium carbonate is one of the reaction that occurs during setting of lime mortar, which was used in the construction of old buildings
Application-2: It has been observed that a white precipitate is formed when aqueous solution of sodium hydroxide is preserved for longer times in the containers which are not closed properly. It is because of the formation of insoluble NaHCO3 when NaOH reacts with excess of carbon dioxide in air.
| NaOH | + | CO2 | <------> | NaHCO3 | ||
| excess | sparingly soluble
in cold water |
Physical state:
* Carbonates and bicarbonates are solids at room temperature. Carbonates of group-1 and group-2 elements are colorless. Whereas, the carbonates of transition elements may be colored.
* The polarizing power of the group-1 metal ions (M+) is less than the polarizing power of group-2 metal ions (M2+). Hence group-2 carbonates are more covalent than the carbonates of group-1.
Whereas the polarizing power decreases down the group with increase in the size of metal ion. Hence the ionic nature increases down the group.
* NaHCO3 and KHCO3 can exist in the solid state. But the bicarbonates of group-2 elements are only known in aqueous solutions.
Solubility in water:
* Except Li2CO3, The group-1 carbonates are fairly soluble in water. The solubility increases down the group as the ionic nature nature increases.
* Group-2 carbonates are sparingly soluble in water as their lattice energies are higher (it is due to increase in covalent nature). There is no clear solubility trend observed down this group.
But group-2 carbonates are soluble in a solution of CO2 due to formation of HCO3-.
Thermal stability:
* Carbonates are decomposed to carbon dioxide and oxide upon heating. Whereas bicarbonates give carbonate, water and carbon dioxide.
* Thermal stability of group-1 and group-2 carbonates (also of bicarbonates) increases down the group as the polarizing power of the metal ion decreases.
* Due to same reason, carbonates of group-1 are more stable than those of group-2.
* Small and highly charged metal ions possess more polarizing power and hence facilitates the decomposition of carbonate ion into carbon dioxide and oxide ion.
The most important reaction shown by these anions is 'decomposition' by liberating carbon dioxide either upon heating or by adding acids. Water or oxide are the other products.
2HCO3- --------> CO32- + CO2 + H2O (upon heating)
HCO3- + H+ --------> CO2 + H2O (in acidic medium)
CO32- --------> CO2 + O2- (upon heating)
CO32- + 2H+ ---------> CO2 + H2O (in acidic medium)
Illustrations:
i) 2NaHCO3 --------> Na2CO3 + CO2 + H2O (upon heating)
ii) NaHCO3 + H+ --------> Na+ + CO2 + H2O
Application: That is why baking soda (NaHCO3) is used as leavening agent to raise cookies, cakes etc.,. It is decomposed to CO2 and water upon heating. This makes the cookies porous and palatable.
iii) Ca(HCO3)2 --------> CaCO3 + CO2 + H2O (upon heating)
Mg(HCO3)2 --------> MgCO3 + CO2 + H2O (upon heating)
Application: Temporary hardness of water is due to presence of bicarbonates of Ca and Mg. It is possible to remove temporary hardness by boiling water. Upon boiling, the soluble bicarbonates are decomposed to insoluble carbonates, which can be filtered off.
iv) CaCO3 --------> CaO + CO2 (upon heating)
Application: This reaction is used to get quick lime (CaO), in lime kilns, which is further used in the preparation of slaked lime, Ca(OH)2. This is also one of the reaction occurring in the manufacture of Portland cement. Technically this type of reaction is called calcination.
v) CaCO3 + 2HCl --------> CaCl2 + H2O + CO2
vi) MgCO3 + 2HCl --------> MgCl2 + H2O + CO2
or in general
CO32- + 2H+ ---------> CO2 + H2O
Comment: This reaction is the principle involved in the detection of carbonate ion present in a given salt.
Calcium carbonate is present in the marble stone. This is decomposed to carbon dioxide when come into contact with acids. Hence the effervescence is observed when acids are dropped on the floor. Lime juice contains citric acid, which liberates carbon dioxide and forms insoluble calcium citrate, which appears as white marking.
Note: Effervescence is observed sometimes on granite floor which may rarely contain carbonates. This may be originated from lichens lived on them.
STUDY OF SOME INDIVIDUAL CARBONATES AND BICARBONATES
Li2CO3:
* Lithium carbonate is a colorless salt with polymeric nature.
* It is sparingly soluble in water and its solubility decreases with increase in temperature. But it dissolves in presence of carbon dioxide due to the formation of LiHCO3.
* It is used in psychiatry to treat mania. The lithium ions interfere the sodium pump and inhibit the activity of protein kinase C (PKC).
* It is also used in the preparation of lithium cobalt oxide - which is present in lithium ion battery cathodes.
Na2CO3:
* Sodium carbonate is a colorless salt.
* It is fairly soluble in water.
* It is also called as washing soda.
* It is used mainly in laundries and in softening hard water.
* It is also used in making glass.
| < Previous | Carbon group: TOC | Silicates-1 > |