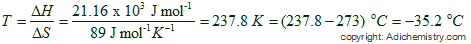
MOLAR ENTROPY - ENTHALPY - GIBBS FREE ENERGY
1) 237800 K
2) 273.8 oC
3) 35.2 K
4) -35.2 oC
Logic:
During vaporization, both liquid and vapor of HI are at equilibrium with each other and hence ΔG is zero.
The relation between ΔG, ΔH and ΔS is given by:
ΔG = ΔH - TΔS
At equilibrium:
0 = ΔH - T ΔS
or
TΔS = ΔH
or
T = ΔH/ΔS
From above equation it is possible to calculate the temperature at which ΔG becomes zero.
ΔvapH = 21.16 kJ mol-1 = 21.16 x 103 J/mol
ΔvapS = 89.0 J K-1 mol-1
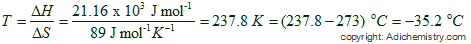
Note: Entropy is usually expressed in J K-1 mol-1.
Conclusion:
Take care of units. You may be tempted to tick for option 3. It is given in oC.
1) What is special about this temperature? What is it known as?
2) Is it possible to calculate ΔGo value for above process? What is the extra information required to do so?