Click on the questions to get solutions
1) It is a second order reaction.
2) Half life is independent of initial concentration of A.
3) The unit of the specific rate, k is L mol-1 s-1.
4) Half life is inversely related to the initial concentration of A.
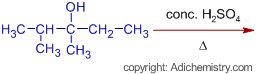
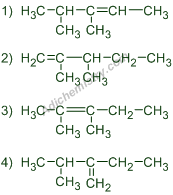
2) The expected major product formed in the following dehydration reaction is:
3) The correct order of ∠FMF bond angles (where M is the central atom) in NSF3, SiF4 and POF3 is:
1) NSF3 > SiF4 > POF3
2) SiF4 > NSF3 > POF3
3) NSF3 > POF3 > SiF4
4) SiF4 > POF3 > NSF3
1) Exhibits optical activity.
2) The metal, M undergoes sp3 hybridization.
3) Can show geometrical isomerism.
4) Its aqueous solution exhibits electrical conductivity.
1) 237800 K
2) 273.8 oC
3) 35.2 K
4) -35.2 oC
7) The incorrect statement regarding the element Ununseptium is:
a) It belongs to group of halogens.
b) It belongs to 7th period of periodic table:
c) It is a radioactive element.
d) It is a strong metal.
8) The correct IUPAC name of ![]() is:
is:
a) 3,4,9-Trimethyldecane
b) 2-Ethyl-3,7-dimethylnonane
c) 2,7,8-Trimethyldecane
d) 3,4,9-Trimethylnonane
I will add next question tomorrow. Please visit again.