1) NSF3 > SiF4 > POF3
2) SiF4 > NSF3 > POF3
3) NSF3 > POF3 > SiF4
4) SiF4 > POF3 > NSF3
Logic:
* First write the Lewis dot structures for the molecules and find the number and types of electrons pairs; magnitude of repulsions between them to arrive at the relative bond angles.
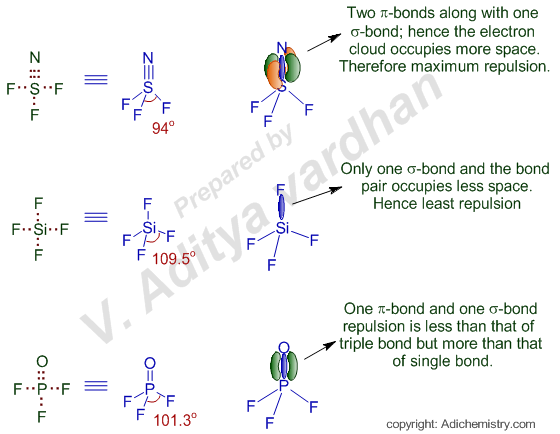
It is known that, from VSEPR theory, the repulsion increases with increase in the volume occupied by electron pair(s). Since the electron density in triple bond occupies more space, it exerts more repulsion than that of double bond and which turn exerts more repulsion than that of single bond.
In NSF3, there is a triple bond between N and S. Hence the repulsions will be more on S-F bond pairs. This will reduce the bond angle more than in other cases.
The bond angle is least affected in case of SiF4, since all the Si-F bonds are single bonds, which exert less repulsion on other bond pairs. Hence the bond angle is maximum i.e. 109o28'
In POF3, there is a double bond between P and O, which also causes more repulsion than single bond, but less than the triple bond. Hence the bond angle will be medium in this case.

1) Write the complete Lewis dot structures of above molecules indicating electrons pairs on atoms connected to central atom.
2) What will be the bond angle in PF3 less than in case of OPF3 or greater than it? Hint: There is now lone pair on P.
| < Previous question | Next question > |