
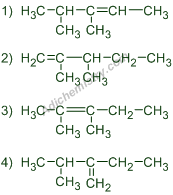
Answer:
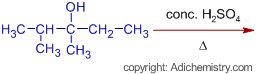
* Alcohols undergo dehydration (elimination of water molecule) in acidic medium to furnish olefins. A double bond is formed due to loss of water molecule. It is an elimination reaction. According to Saytzeff's rule (also known as Zaitsev's rule), during dehydration, more substituted alkene (olefin) is formed as a major product, since greater the substitution of double bond greater is the stability of alkene.
* The IUPAC name of the alcohol undergoing dehydration reaction is 2,3-dimethylpentan-3-ol.
* Sulfuric acid is a strong acid and ionizes to give a proton.
![]()
* Thus formed H+ ion attacks the -OH group. The OH2+ group formed is a good leaving group due to the accumulation of positive charge on the oxygen atom. Now the loss of H2O creates a positive charge on the carbon atom and thus by forming a tertiary carbocation.
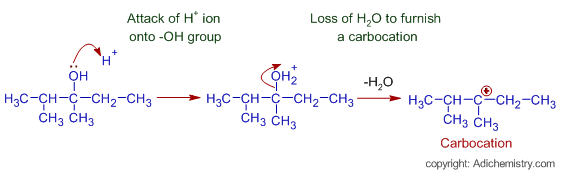
* Finally, one of the hydrogen, adjacent to the positively charged carbon, is removed as a proton, H+ to make a double bond. However, there are three adjacent hydrogen atoms (indicated by descriptors I, II & III). Hence three different alkenes are possible.
However according to Saytzeff's rule, highly substituted alkene, as shown below by the loss of H+(I), is formed as a major product (referred to as Saytzeff's product)
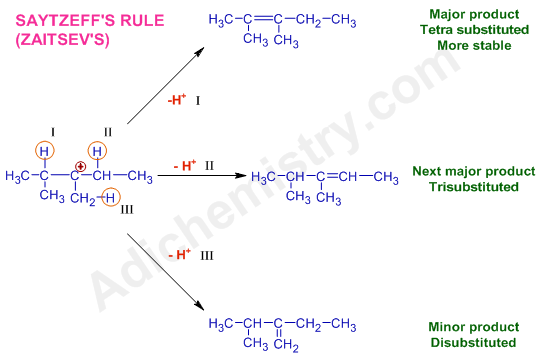
Note: Since a tertiary carbocation is formed during the reaction, the scope of rearrangement by alkyl shift is not observed.
Question-1) Alkenes upon reaction with BH3 and oxidation with H2O2 in basic medium form alcohol according to:
1) anti markownikoff's addition
2) markownikoff’s addition
3) saytzeff’s rule
4) none of these
Answer: 1
Question-2) Draw the Zaitsev product formed when 2,3-dimethylpentan-3-ol undergoes an E1 dehydration.
(or)
Draw the major product of the following alcohol dehydration in the presence of H2SO4. Use the zaitsev rule (no rearrangements) to predict the major product when necessary. Include all hydrogen atoms.

Answer: Already discussed.
Question-3) According to which rule, more substituted alkene is obtained as major product in an elimination reaction?
1) Markownikoff's rule
2) Saytzeff'f rule
3) Bredt's rule
4) Karasch rule
Answer: 2
1) What is Saytzeff's rule? Write the statement (definition). Illustrate with a suitable example.
Answer: Simple definition - More substituted alkene (olefin) is formed as a major product during elimination reactions. It is also called Zaitsev's rule.
Note: Present case as explained on this page is the best example of mechanism of Saytzeff rule in organic chemistry using dehydration alcohol.
2) What is Saytzeff's product? What is the mechanism of formation of stable alkene by elimination?
3) How do we get alkene if a halogen is present instead of -OH group? Does this reaction too follow Saytzeff's rule?
Answer: A strong base is required to remove proton on the beta carbon, which is followed by removal of halogen with concomitant formation of a double bond. It may follow E1 or E2 mechanism. One of the side reaction is nucleophilic substitution. Yes, as it is the elimination (dehydrohalogenation) reaction, it follows Saytzeff rule provided the substituents are alkyl groups and are not excessively bulky. We cannot say anything straightforward when bulkier groups and other substituents are present.
4) Why do more substituted alkenes are more stable?
Answer: The alkenes with greater number of alkyl substituents are more stable due to hyperconjugation.
5) What are the IUPAC names of alkenes formed in the above reaction?
Answer: I) 2,3-dimethylpent-2-ene II) 3,4-dimethylpent-2-ene III) 2-methyl-3-methylidenepentane
6) Write a chemical reaction to illustrate saytzeff’s rule. Explain with example.
7) What is set jeff rule?