1) Exhibits optical activity.
2) The metal, M undergoes sp3 hybridization.
3) Can show geometrical isomerism.
4) Its aqueous solution exhibits electrical conductivity.
Since the complex does not give any precipitate with silver nitrate and the metal belongs to d-block, we can conclude that both Br- and Cl- ions are involved in the dative bond formation with the metal and hence are not ionizable. Hence it cannot exhibit electrical conductivity in aqueous medium.
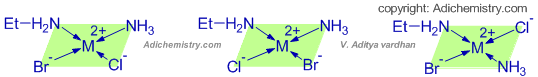
Note: No ions are formed when this complex is dissolved in water. It is a neutral coordination complex and can be represented as: [M(NH3)(EtNH2)BrCl]
This complex can take either tetrahedral or square planar geometry. Theoretically both are possible.
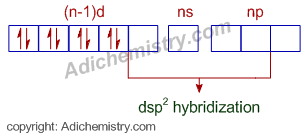
As such the complex is diamagnetic; indicating all the electrons in the (n-1)d orbital are paired up. Hence, according to VBT model, the metal should undergo dsp2 hybridization and the complex is square planar that has a plane of symmetry. Hence it wont show optical activity.

Why all the electrons are paired up? It is due to presence of strong field ligands which force the electrons to pair up.
Note: If the metal is sp3 hybridized, the complex will be tetrahedral and can show optical activity.
However, it can show geometrical isomerism as shown below.
1) How do you arrive at the oxidation number of metal ion in the complex?
2) To which group of d-block, the metal belongs to?
3) What is the magnetic moment of complex if it is tetrahedral?
4) State whether this complex is inner orbital complex or outer orbital one?
5) Whether the pairing energy, PE of electrons is greater or the crystal field splitting energy, Δ for this complex?
6) What is the IUPAC name of this compound?
| < Previous question | Next question > |