(JEE MAIN 2006)
a) 25h/π
b) 1.0h/π
c) 10h/π
d) 2.5h/π
Logic:
The angular momentum (mvr) of electron in nth orbit is equal to nh/2π.
Solution:
For the electron in 5th orbit,
angular momentum = mvr = 5h/2π = 2.5h/π
Conclusion:
Correct option is "d".
Extra information
Note: nh/2π gives angular momentum of electron revolving in a circular orbit as proposed by Neils Bohr. However, the exact orbital angular momentum of electron in a given orbital is calculated by using the following equation.
![]()
(IIT JEE-1996)
a) 0.5h/π
b) 0
c) h/2π
d) √2h/π
Solution:
![]()
For s orbital, l = 0.
Therefore:
orbital angular momentum = 0.
(IIT JEE - 1997)
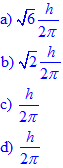
Solution:
Since for a d-electron, l = 2,
![]()
3) According to Bohr’s theory, which one of the following values of angular momentum of hydrogen atom is not permitted ?
(EAMCET 2011-Medical)
a) 0.5h/π
b) 1.25h/π
c) h/π
d) 1.5h/π
Theory:
Bohr's atomic model is based on quantum theory, according to which, only definite values of angular momentum are possible for electron revolving in a circular orbit. It is given by mvr = nh/2π.
The principal quantum number, n can have only integral values i.e., 1, 2, 3, 4............
Solution:
| Option | Angular momentum | n value | Permitted or not? |
| a) | 0.5h/π = 1 x h/2π | 1 | yes |
| b) | 1.25h/π = 2.5 x h/2π | 2.5 | no |
| c) | h/π = 2 x h/2π | 2 | yes |
| d) | 1.5h/π = 3 x h/2π | 3 | yes |
Conclusion:
Answer to the question is "b".
(EAMCET 2006-Medical)
a) Third orbit
b) Second orbit
c) Fourth orbit
d) Fifth orbit
Solution:
1.5h/π = 3 x h/2π
Therefore n = 3 and the electron is in the third orbit of excited state.
(EAMCET 2010-M)
a) 3h/2π
b) h/2π
c) h/π
d) 2h/π
Logic:
First find out the n value for the orbital using equation:
![]()
Then calculate the angular momentum using the formula:
mvr = nh/2π
Solution:
Radius of orbit (r) = 0.529 x n2/Z Å
or
4.768 Å = 0.529 x n2/1 Å
or
n2 = 4.768 / 0.529 = 9.013
Therefore,
n = 3
And the angular momentum, mvr = 3h/2π
(CSIR UGC NET DECEMBER 2012)
1) inter-electron repulsion
2) spin-orbit interaction
3) hyperfine coupling
4) external magnetic field.
Explanation:
The strong inter electron repulsion in many electron atoms results in coupling of individual orbital angular momenta and spin angular momenta. Therefore the total orbital angular momentum (L) and total spin angular momentum (S) are good quantum numbers instead of individual values.
A) The energy of the bound electron is determined by spin quantum number, s.
B) The azimuthal quantum number ‘l’ specifies the magnitude of orbital angular momentum of electron.
C) Energy of the electron is determined by 'l'.
D) Every orbital has same angular momentum.
Answer: B
(BANARUS HINDU UNIVERSITY BHU MSC 2011)
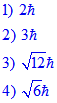
Explanation:
For n = 4, the possible 'l' values are 0, 1, 2 & 3.
The z-component of L is given by,
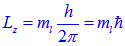
For maximum possible 'l' value i.e 3 and for this ml can have values -3, -2, -1, 0, +1, +2, +3.
Substitute the maximum ml value to get the answer.
Answer: 2
1) What are the orbital angular momentum values of electrons in 2p and 3p orbitals in hydrogen atom?
2) What is the difference between angular momentum values of ground and 1st excited states of hydrogen atom?
3) What is the angular momentum of electron in 3d orbital?
Jump to Relation between Energy & Wavelength of photon - IIT JEE - NEET - IT JAM solved problems