(IIT JEE 1985)
a) toluene
b) benzene
c) benzoic acid
d) nitrobenzene
Logic:
Electrophilic nitration involves attack of nitronium ion on benzene ring. The reactivity of benzene ring increases with increase in the electron density on it. The group which increase the electron density on the ring also increase the reactivity towards electrophilic substitution. These groups which show +I or +M effect like alkyl, alkoxy etc., release electrons towards benzene ring.
Conclusion:
Toluene is more reactive towards electrophilic nitration due to presence of electron donating methyl group.
(IIT JEE 1986)
a) benzoyl chloride
b) m-chlorotoluene
c) benzyl chloride
d) o- and p-chlorotoluene
Logic & Solution:
In presence of FeCl3 and in dark, electrophilic substitution on benzene ring by Cl+ ion at ortho and para positions occurs to give o- and p-chlorotoluene.
Since methyl group activates the ring at ortho and para positions more than meta position, the electrophilic substitution occurs mostly at these positions. Again, amongst these, para isomer is the major product due to steric reasons.
Conclusion:
Correct option is 'd'.
(IIT JEE 1999)
a) is para directing
b) is meta directing
c) activates the ring by hyperconjugation
d) deactivates the ring
Logic & Solution:
The methyl group activates the ring at ortho and para positions by both hyperconjugation as well as through positive inductive effect.
Conclusion:
Correct options are: 'a' and 'c'.
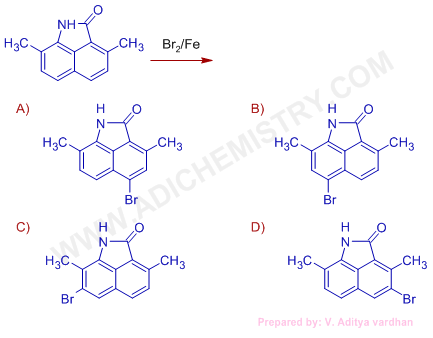
(IIT JEE 2004)

Logic & Solution:
Among -CH3 and -NH groups, the -NH group is strongly activating. It activates both ortho and para positions to it. However, the ortho positions are blocked (already substituted). Hence the bromination occurs at para position.
The C=O group is ring deactivating group.
Conclusion:
Hence the major product is that shown under option 'B'.
1) Write the mechanism of nitration?
2) Write the formation of Br+ ion from Br2/Fe.
3) What is hyperconjugation?
| < Previous question | Next question > |